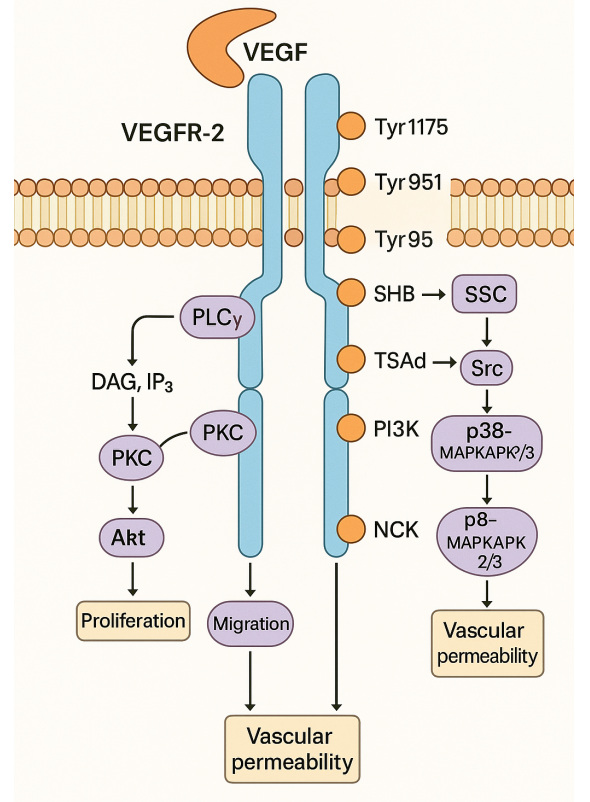
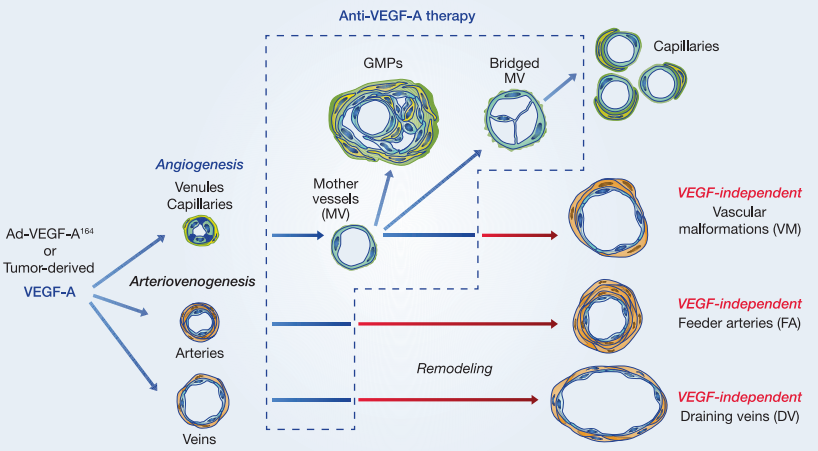
Covid-19 Inhibitor Drug Discovery
Abstract
In just 20 years, Coronaviruses have caused three pandemics of infectious diseases including COVID-19, severe cute respiratory syndrome coronavirus (SARS CoV), and Middle east respiratory syndrome coronavirus (MERS CoV). So far, there is no specific drug specifically for the prevention and treatment of coronavirus.
1. COVID-19 and Viral Research Applications
In just 20 years, Coronaviruses have caused three pandemics of infectious diseases including COVID-19, severe cute respiratory syndrome coronavirus (SARS CoV), and Middle east respiratory syndrome coronavirus (MERS CoV). So far, there is no specific drug specifically for the prevention and treatment of coronavirus. In the absence of vaccines or specific antiviral drugs during a virus outbreak, non-specific therapeutic interventions are usually used to prevent severe complications and reduce severe morbidity and mortality, such as the provision of supportive care, including adequate rest, rehydration, and painkillers. At the same time, traditional Chinese medicine, hormone drugs, broad-spectrum antibiotics, antivirals, antifungal agents and interferon-α2b can be used to minimize the risk of co-infection with pathogens. Researchers around the world are taking a hard look at SARS-CoV-2, the coronavirus that caused the COVID-19 pandemic. The response to this global emergency requires a concerted effort by all to acquire knowledge and tools at a faster pace. For researchers, accurate and timely supplies of tools and raw materials are essential. As a science service, MuseChem provides research chemicals and pharmaceutical standards for COVID-19 to major institutions and platforms around the world.
2. Related drugs based on viruses
2.1 Nucleic acid synthesis inhibitors
Remdesivir (RDV, GS-5734) is a nucleoside analogue. On October 22, 2021, the U.S. Food and Drug Administration (FDA) approved Gilead Sciences’ antiviral drug Remdesivir for the treatment of hospitalized COVID-19 patients, becoming the first officially approved treatment for COVID-19 in the United States. It can play a therapeutic effect by inhibiting the synthesis of viral nucleic acid and has antiviral activity. Gilead believes that antiviral nucleic acid analogs such as ribavirin will be cleaved out by ExoN of coronavirus when integrated into viral RNA in the treatment of coronavirus infection, but RDV is resistant to ExoN, which leads to more significant efficacy of RDV in the treatment of coronavirus than other nucleic acid drugs. Previously, RDV has been studied mainly as an experimental drug against the Ebola virus, which has shown strong anti-filovirus efficacy in vitro.
Molnupiravir (EIDD-2801/MK-4482) is an oral precursor of β-D-N4-hydroxy cytidine (NHC), a ribonucleoside with broad antiviral activity against RNA viruses. The uptake of NHC by viral RNA-dependent RNA polymerases leads to viral mutations and lethal mutagenesis. Molnupiravir was approved for marketing by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) in November 2021 for the treatment of adult patients with mild to moderate COVID-19 who are critically ill and at high risk of hospitalization. Molnupiravir became the world’s first oral anti-coronavirus drug approved for the treatment of mild to moderate COVID-19 in adults. Molnupiravir was found to reduce viral loads rapidly and, if given early, may also reduce infectivity in treated cases.
2.2 Protease inhibitors
2.2.1 Darunavir (Prezista), a second-generation HIV-1 protease inhibitor, was first marketed in the United States in July 2006. Developed by Tibotec, a Johnson & Johnson subsidiary, Darunavir can be used in combination with ritonavir and other retroviral drugs to treat HIV infection. Studies have found that it has a certain inhibitory effect on the novel coronavirus. In vitro cell experiments showed that darunavir could significantly inhibit virus replication at the concentration of 300μmol·L-1, and the inhibition efficiency was up to 280 times as compared with the untreated group.
2.2.2 TypeⅡ Transmembrane Serine Proteases (TMSPSS2) inhibitors may be used to block COVID-19 infection and then treat COVID-19. ACE2 is a metallopeptidase expressed in major viral target cells, including type Ⅱ pulmonary cells and intestinal epithelial cells. Its catalytic domain binds to the S protein of SARS CoV with high affinity. Cleavage of S protein by host cell proteases is critical for viral infectivity. TMSPSS2 can activate SARS spike protein by lysis of spike protein on the cell surface, and spike protein can then combine with ACE2 to enter host cells. TMPRSS2 is expressed in ACE2-positive cells in the human lung. Cell experiments indicated that TMPRSS2 may play an important role in the spread of SARS CoV in the human respiratory tract. Camostat mesylate was developed by Ono Pharmaceutical Industrial Research Institute in Japan and marketed in January 1986. As a mature drug, its inhibitory effect on COVID-19 is still under study.
2.3 RNA polymerase inhibitors
Favipiravir (Avigan) is a broad-spectrum antiviral drug that can selectively inhibit the RNA polymerase of the influenza virus. In March 2014, Favipiravir was approved for the antiviral treatment of influenza A and B in Japan. In addition to its anti-influenza activity, Favipiravir blocks the replication of many other RNA viruses, including alphaviruses, flaviviruses (yellow fever and West Nile viruses), enteroviruses (poliovirus and rhinovirus), respiratory syncytial viruses, and noroviruses. In February 2020, several drugs, such as Favipiravir, were indicated by relevant institutions to have anti-coronavirus activity, and subsequent studies need to be further verified by animal experiments and clinical trials.
2.4 Membrane fusion inhibitors
Arbidol (Umifenovir), developed by the Center for Medicinal Chemistry of the former Soviet Union, is a synthetic broad-spectrum antiviral compound for the prevention and treatment of human influenza A and B infections and post-influenza complications. It has been used as an anti-influenza virus drug in many countries for decades. Arbidol is active against many DNA/RNA and enveloped/nonenveloped viruses and inhibits membrane fusion between viral particles and plasma membranes and between viral particles and endosomal membranes by embedding in membrane lipids. The data showed that Arbidol at the concentration of 10-30 μmol·L-1 could effectively inhibit the coronavirus up to 60 times compared with the control group without drug treatment, and significantly inhibited the pathological effect of the virus on cells. The effect of subsequent treatment needs to be further verified.
2.5 Natural product inhibitors
Resveratrol is a natural compound found in grape seeds, skins, and red wine. Resveratrol can inhibit infections caused by multiple pathogens and has been shown to inhibit a variety of human viruses, including the influenza virus, herpes simplex virus, respiratory syncytial virus, HIV-1, varicella zoster virus, enterovirus 71, human metapneumovirus, human rhinovirus 16, polyomavirus and cytomegalovirus. Resveratrol can be used as an adjunctive treatment for respiratory infections because of its antiviral activity against respiratory viruses and its anti-inflammatory effect. Resveratrol inhibits viral replication, viral protein synthesis, gene expression, and nucleic acid synthesis by down-regulating inflammatory signal transduction, so it has a wide range of antiviral effects.
3. Host-based drugs
3.1 Interferon and inducer drugs
3.1.1 Polyinosinic: Polycytidylic acid (poly(I: C)), a synthetic analog of dsRNA strongly induces type I interferons, although studies have shown that the 4a protein of MERS-CoV contains the ability to interact with polyinosinic acid: The PCA-interacting double-stranded RNA-binding domain inhibited PCA-or Sendai virus-induced interferon production, but experiments showed that PCA still greatly reduced MERS-CoV load in BALB/c mice.
3.1.2 Nitazoxanide is a nitrothiacylamide derivative, used for the treatment of cryptosporidium, Giardia, and amoeba caused by diarrhea and cryptosporidiosis associated with AIDS patients. The drug was first marketed in Mexico in 1996 to fight parasitic infections. In 2002, ALINIA(Nitazoxanide) was approved for sale in the United States. Studies have shown that Nitazoxanide does not inhibit the transcription of viral RNA or prevent the synthesis of viral proteins, but may interfere with the processing of post-transcriptional proteins and the assembly of viral glycoproteins. Another possible mechanism is that the salicylic acid ring in Nitazoxanide inhibits the synthesis of cyclooxygenase, which in turn inhibits the replication of coronavirus.
3.2 Glycosylation inhibitors
Chloroquine, an anti-malarial and anti-inflammatory agent widely used to treat malaria and rheumatoid arthritis, has recently been reported as a potential broad-spectrum antiviral agent. Chloroquine can block viral infection by upregulating the endosomal pH required for viral fusion with cells and inhibiting the glycosylation of cell receptors. Chloroquine can specifically interact with glycosyl-modifying enzymes or glycosyltransferases in human cells. Recent cellular assays have shown that chloroquine is highly effective in controlling SARS-CoV-2 infection in vitro, acting in both the entry and post-entry phases of SARS-CoV-2 infection in VeroE6 cells. In addition to its antiviral activity, chloroquine has an immunomodulatory activity that synergistically enhances its antiviral effects in vivo. Chloroquine is a drug that has been used for more than 70 years, and it has a good chance of being clinically appropriate for COVID-19.
3.3 Inhibitors of endocytosis
Chlorpromazine is an antipsychotic drug used to treat schizophrenia. Chlorpromazine was launched in France in 1952 under the trade name Largactil. It was approved by the FDA in 1957 and marketed under the trade name Thorazine. Most viruses, after attaching to host surface receptors, use clathrin-dependent and clathrin-independent pathways to enter cells. SARS used clathrin-dependent mechanisms to enter host cells. Chlorpromazine, an inhibitor of clathrin-dependent endocytosis, can significantly inhibit the replication of SARS-CoV. Chlorpromazine was found to be a broad-spectrum viral inhibitor that inhibited HCV, αvirus, and a variety of coronaviruses including human coronavirus 229E, SARS-CoV, and MERS-CoV in vitro.
3.4 Kinase inhibitors
Imatinib (Gleevec) is a small-molecule inhibitor developed by Nobartis in 2001 and first marketed in the United States for the treatment of chronic myeloid leukemia and malignant gastrointestinal stromal tumors. Imatinib is specifically designed to target Abl2 kinase(Abelsontyrosine-proteinkinase2), a nonreceptor tyrosine kinase present in the nucleus and mitochondria that mediates multiple cellular processes, from embryonic morphogenesis to viral infection. The Abl2 kinase is essential for SARS-CoV and MERS-CoV replication in vitro. In vitro experiments showed that imatinib, as an in vitro inhibitor of SARS-CoV and MERS-CoV, could significantly reduce the titers of SARS-CoV and MERS-CoV and inhibit the internalization process of SARS-CoV and MERS-CoV pseudotype virions. The anti-coronavirus activity of imatinib is mainly manifested in the early stage of infection, mainly by inhibiting the fusion of virions on endosomal membranes.