Suzuki-Miyaura Reaction
The Suzuki-Miyaura reaction is a type of cross-coupling reaction in organic chemistry. It was first described by Akira Suzuki and his colleague, Norio Miyaura, in 1979. This reaction involves the coupling of an organoboron compound, usually an aryl boronic acid or its ester, with an organic halide or pseudohalide, such as an aryl chloride or bromide. The Suzuki-Miyaura reaction is catalyzed by a palladium complex, often in the presence of a base (such as a carbonate or a phosphine). The reaction typically takes place in an organic solvent such as tetrahydrofuran or dimethylformamide. The Suzuki-Miyaura reaction is widely used in organic synthesis for the formation of carbon-carbon bonds. It is a very useful method for the synthesis of biaryl compounds, which are important in many fields including pharmaceuticals, materials science, and agrochemicals.
Importance of Suzuki-Miyaura reaction
The Suzuki-Miyaura cross-coupling reaction is a widely used synthetic method in organic chemistry that allows for the formation of carbon-carbon bonds between aryl or vinyl halides and boronic acids or boronate esters. This reaction was first reported by Akira Suzuki and N. Miyaura[1] in the early 1980s and has since become one of the most versatile and powerful tools in modern organic synthesis(Scheme 1).
![]()
Scheme 1 General scheme of Suzuki reaction
The Suzuki-Miyaura cross-coupling reaction has many advantages, the first of which is its wide range of substrate scope. This means it can be used for the preparation of various functionalized compounds, including natural products, pharmaceuticals, and materials, with great efficiency and selectivity. It can also apply to the complex compounds with various functional groups such as esters, ketones, and nitriles[2]. A palladium complex typically catalyzes the Suzuki-Miyaura cross-coupling reaction, facilitating the transmetallation between the aryl or vinyl halide and the boronic acid or boronate ester. A ligand is usually present to control the reactivity and selectivity of the reaction. Besides its significance in organic synthesis, the reaction has also been founded applications in materials science. Conjugated polymers that have potential applications in organic electronics, optoelectronics, and photovoltaics can be prepared using this reaction[3]. The reaction has also been used to functionalize carbon-based materials such as carbon nanotubes and graphene, leading to the development of new materials with customized properties.
Overall, the Suzuki-Miyaura cross-coupling reaction is an essential tool for modern organic synthesis and materials science. Its broad substrate scope, high selectivity, and compatibility with functional groups make it a valuable synthetic method for the preparation of a wide range of compounds.
Mechanism of Suzuki Reaction
The Suzuki-Miyaura cross-coupling reaction is a palladium-catalyzed reaction that involves the formation of a carbon-carbon bond between an aryl or vinyl halide and a boronic acid or boronate ester. The reaction mechanism can be divided into several steps, including oxidative addition, transmetallation, and reductive elimination[4].
The first step of the reaction mechanism is oxidative addition, which involves the coordination of the palladium catalyst to the aryl or vinyl halide substrate. This step is typically rate-limiting and requires the presence of a suitable ligand to stabilize the intermediate palladium complex. Commonly used ligands include phosphines, phosphites, and N-heterocyclic carbenes.
The second reaction step is transmetallation, where ligands exchange between the palladium complex and the boronic acid or boronate ester substrate. The electron-rich boron atom coordinates with the palladium complex, enabling the formation of the new carbon-carbon bond. It’s the key step of this reaction.
The final reaction step is reductive elimination, where the palladium-carbon bond breaks, and the carbon-carbon bond forms. A ligand can stabilize the intermediate palladium complex and promote the reductive elimination reaction (Figure 1).
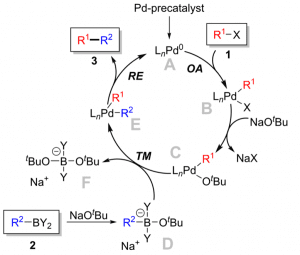
Figure 1 Mechanism of Suzuki Reaction
The role of the palladium catalyst and ligand in the Suzuki-Miyaura cross-coupling reaction is critical for the success of the reaction. The palladium catalyst serves as a key mediator for the transmetallation and reductive elimination steps, and its reactivity can be tuned by the choice of ligand. Different ligands can have a significant impact on the rate, selectivity, and scope of the reaction. For example, phosphine ligands such as PPh3 are commonly used for the Suzuki-Miyaura cross-coupling reaction, as they can promote rapid oxidative addition and transmetallation. However, they may also lead to the formation of unwanted side products or require harsh reaction conditions.
In addition to the choice of catalyst and ligand, the substrate scope of the Suzuki-Miyaura cross-coupling reaction is an important consideration. The reaction can be used to form carbon-carbon bonds between a wide range of aryl or vinyl halides and boronic acids or boronate esters. However, certain functional groups or steric hindrances can limit the effectiveness of the reaction. For example, the presence of electron-withdrawing groups on the aryl or vinyl halide substrate can decrease the reactivity of the substrate, while bulky or sterically hindered boronic acid or boronate ester substrates may require longer reaction times or higher temperatures.
Overall, the Suzuki-Miyaura cross-coupling reaction is a powerful synthetic tool for the formation of carbon-carbon bonds between aryl or vinyl halides and boronic acids or boronate esters. The reaction mechanism is highly dependent on the choice of palladium catalyst and ligand, and the substrate scope can be influenced by factors such as functional groups and steric hindrances. However, with careful optimization and selection of reaction conditions, the Suzuki-Miyaura cross-coupling reaction can be used to synthesize a wide range of functionalized compounds with high efficiency and selectivity.
Recent Advances in Catalysts and Ligands
Recent advances in catalysts and ligands have greatly expanded the scope and utility of the Suzuki-Miyaura cross-coupling reaction. Palladium-based catalysts remain the most commonly used for this reaction, but novel catalysts and ligands have been developed that offer improved reactivity, selectivity, and scalability.
Recent research has focused on developing stable and efficient palladium catalysts for the Suzuki-Miyaura cross-coupling reaction. NHC ligands and biarylphosphines have been discovered as novel ligands that can improve the reaction’s reactivity and selectivity. Another area of study[5,6] has been ligand-free catalysts such as Pd(OAc)2, Pd(0), and Pd(OAc)2/PhSiH3, which have shown good efficiency and selectivity.
Non-palladium catalysts like copper have also been explored for the cross-coupling reaction, particularly for coupling aryl halides and boronic acids. Although copper-catalyzed reactions require milder conditions and lower catalyst loadings than palladium-catalyzed reactions, their selectivity can be more challenging to control.
Other non-palladium catalysts that have been explored for the Suzuki-Miyaura cross-coupling reaction include nickel, copper, and iron-based catalysts[7-10]. For example, nickel-catalyzed reactions have been shown to be effective for the coupling of aryl halides and boronic acids under mild reaction conditions. Iron-based catalysts have also been explored, with Fe(acac)3 and FeCl3 found to be effective for the coupling of aryl bromides and boronic acids(Table 1).
Table 1 List of common catalyst and ligand of Suzuki reaction
| Pd(PPh3)4 | Pd(OAc)2 | Pd2(dba)3 | PdCl2 |
| Pd(PhCN)2Cl2 | Pd(PhCN)4Cl2 | Pd(OAc)2/AsPh3 | Pd(OAc)2/DPPE |
| Pd(OAc)2/dppf | Pd(OAc)2/dppb | Pd2(dba)3/dppf | Pd(OAc)2/SPhos |
| Pd(OAc)2/XPhos | Pd(OAc)2/Tol-BINAP | Pd(OAc)2/BrettPhos | Pd(OAc)2/JohnPhos |
| Pd(OAc)2/QUINOX | Pd(OAc)2/BIPHEP | Pd(OAc)2/XANTPHOS | Pd(OAc)2/MeOBIPHEP |
| Pd(OAc)2/dm-SEGPHOS | Pd(OAc)2/Sbinap | Pd(OAc)2/Me-DalPhos | Pd(OAc)2/TBuXPhos |
| Pd(OAc)2/dm-SO-phos | Pd(OAc)2/Tol-TTP | Pd(OAc)2/BINAP | Pd(OAc)2/BPE |
| Pd(OAc)2/Bu-tBrettPhos | Pd(OAc)2/Ph-BPE | Pd(OAc)2/Ph-DalPhos | Pd(OAc)2/MOP |
| Pd(OAc)2/tBu-XylPhos | Pd(OAc)2/SEGPHOS | Pd(OAc)2/SPhos-PEPPSI-IPr | Pd(OAc)2/K4[Fe(CN)6] |
| Pd(OAc)2/BisNHC | Pd(OAc)2/L1 | Pd(OAc)2/Pyridine-Carboxamide Ligands | Pd(OAc)2/(S)-TRIP |
| Ni(COD)2 | NiCl2 | Fe(CO)5 | Fe(acac)3 |
| Co(acac)3 | CoCl2 | RhCl(PPh3)3 | Rh2(OAc)4 |
| RuCl2(PPh3)3 | RuPhos | AuCl3 | AuPPh3 |
| Ag2CO3 | AgOAc | Zn(OAc)2 | ZnCl2 |
| PtCl2(PPh3)2 | PtCl4 | Pd(Bs)2 | Pd(CF3SO3)2 |
Overall, recent advances in catalysts and ligands have greatly expanded the scope and utility of the Suzuki-Miyaura cross-coupling reaction. Novel palladium-based catalysts and ligands have been developed that offer improved reactivity, selectivity, and scalability, while non-palladium catalysts have also been explored as alternatives to traditional palladium-based catalysts. With continued research and development, the Suzuki-Miyaura cross-coupling reaction is likely to remain a critical tool in synthetic chemistry for the construction of complex organic molecules and materials.
Substrate Scope and Applications
The Suzuki-Miyaura cross-coupling reaction is a versatile tool for the synthesis of various organic molecules and materials. By forming a carbon-carbon bond between aryl or vinyl halides and boronic acids, it has enabled the construction of complex organic compounds. The reaction’s widespread use in organic synthesis is evident in the production of many biologically active compounds and natural products. For instance, tamoxifen, a vital breast cancer drug, was first synthesized using the Suzuki-Miyaura cross-coupling reaction[11]. Likewise, celecoxib and raloxifene drugs, as well as natural products such as resveratrol and tetracycline antibiotics, have been synthesized using this reaction.
The reaction has also found application in materials science, particularly in the synthesis of conjugated polymers for use in electronic and optoelectronic devices. The reaction has been used to synthesize a range of polymers, including polythiophenes, polypyrroles, and polyfluorenes, which have been used in applications such as solar cells, light-emitting diodes, and transistors.
In addition to its use in the synthesis of complex organic molecules and materials, the Suzuki-Miyaura cross-coupling reaction has also been applied to the synthesis of molecular probes and imaging agents. For example, the reaction has been used to synthesize fluorescent probes for imaging cancer cells and other biological targets.
The substrate scope of the Suzuki-Miyaura cross-coupling reaction has been continuously expanded in recent years through the development of new catalysts and ligands. For example, aryl chlorides, which were previously considered poor substrates for the reaction, can now be coupled using a variety of palladium catalysts and ligands. The reaction can also be used to couple vinyl halides and triflates, as well as heteroaryl halides, such as pyridines and furans[12].
Despite the broad substrate scope of the reaction, there are some limitations to its use. For example, steric hindrance can limit the reactivity of certain substrates, particularly those with bulky substituents. Additionally, the reaction can be sensitive to air and moisture, and may require careful handling and purification.
In conclusion, the Suzuki-Miyaura cross-coupling reaction is a powerful tool in synthetic chemistry, with a broad substrate scope and wide-ranging applications in organic synthesis, materials science, and molecular imaging. Continued research and development of new catalysts and ligands will likely further expand the scope and utility of the reaction, enabling the synthesis of increasingly complex and diverse organic molecules and materials.