Dulaglutide in Type 2 Diabetes
Abstract
Diabetes presents a formidable challenge to global healthcare systems, steadily increasing its societal and economic burden. Approximately 415 million adults grapple with this condition, predominantly type 2 diabetes (T2D), with a substantial portion unaware of their status. T2D manifests through dysregulated glucose homeostasis, culminating in hyperglycemia, and is linked to significant mortality and morbidity, notably from cardiovascular and renal complications. Effective management, aimed at maintaining glycemic control below 7% HbA1c, is paramount to mitigate these risks. Despite the array of available antihyperglycemic agents, achieving and sustaining optimal glycemic levels remains arduous. Among these agents, subcutaneous once-weekly dulaglutide, a glucagon-like-1 (GLP1) receptor agonist, has gained approval for T2D treatment globally. This review, focusing on its therapeutic efficacy and tolerability, delves into pivotal trials such as REWIND CVOT, elucidating its pharmacological profile and clinical utility in T2D management.
Therapeutic Efcacy of Dulaglutide
In Clinical Trials
The robust clinical experience firmly establishes the glycemic efficacy of subcutaneous once-weekly dulaglutide, whether used alone or in combination with oral antihyperglycemic drugs (OADs) or titrated-to-target basal or prandial insulin, in adults (aged ≥18 years) with inadequately controlled type 2 diabetes (T2D). Recent discussions center on AWARD combination therapy trials, particularly AWARD-7, involving patients with inadequately controlled T2D and moderate to severe chronic kidney disease (CKD). Findings from Japanese trials and AWARD-CHN1 and -CHN2 trials consistently mirror those of pivotal AWARD trials. In pivotal AWARD trials, recommended dulaglutide dosages (0.75 or 1.5 mg once weekly) consistently led to clinically meaningful enhancements in glycemic control across various patient demographics and baseline characteristics. Whether patients had baseline HbA1c levels, age, BMI, gender, race/ethnicity, duration of diabetes, or baseline β-cell function, dulaglutide demonstrated efficacy. Typically, inadequately controlled T2D was defined by baseline HbA1c levels ranging from 7 to 11%. The primary endpoint, the least-squares mean change from baseline in HbA1c, was consistently improved across studies. When used as an add-on therapy to metformin, dulaglutide outperformed sitagliptin in improving glycemic control and reducing body weight. Similarly, as an add-on to a sulfonylurea, dulaglutide significantly enhanced glycemic control compared to placebo, with substantial weight loss observed. Additionally, dulaglutide was superior to titrated-to-target insulin glargine as an add-on therapy, showcasing better reductions in HbA1c levels and a higher proportion of patients achieving target HbA1c levels. In comparisons with other GLP1 receptor agonists, dulaglutide was noninferior to liraglutide, superior to exenatide, and less effective than semaglutide in reducing HbA1c levels. When added to a sodium-glucose co-transporter 2 inhibitor (SGLT2i), dulaglutide showed greater improvements in HbA1c levels than placebo. Furthermore, dulaglutide demonstrated significant improvements in patient-reported outcomes, including quality of life measures, satisfaction with treatment, and perceptions of hyperglycemia. In patients receiving titrated-to-target basal or prandial insulin, dulaglutide consistently improved glycemic control and led to weight loss compared to placebo or insulin glargine. Moreover, dulaglutide showed promising results in patients with CKD, leading to improvements in glycemic control, reduced decline in estimated glomerular filtration rate (eGFR), and decreased albuminuria. Overall, dulaglutide emerges as a versatile and effective treatment option for patients with inadequately controlled T2D, offering substantial benefits in glycemic control, weight management, and patient-reported outcomes across various therapeutic combinations and patient populations.In Real-World Studies
In extensive real-world studies conducted in the US, dulaglutide consistently demonstrated superior glycemic efficacy compared to exenatide or liraglutide when used as an initial GLP1 receptor agonist (GLP1 RA) therapy for adults with type 2 diabetes (T2D). In GLP1 RA-naïve patients, dulaglutide treatment led to significantly greater reductions in HbA1c levels at both 6 and 12 months compared to liraglutide or exenatide. Importantly, adherence to treatment played a pivotal role, with adherent patients experiencing significantly greater reductions in HbA1c across all treatment cohorts. Moreover, in the DISPEL study, which focused on patients initiating their first injectable antihyperglycemic agent (AHA), dulaglutide exhibited superior glycemic control compared to basal insulin at the one-year mark. Dulaglutide recipients demonstrated greater improvements in HbA1c levels and were more likely to achieve target HbA1c levels compared to basal insulin recipients. Additionally, dulaglutide users exhibited higher adherence and persistence to treatment and lower rates of treatment discontinuation compared to those on basal insulin.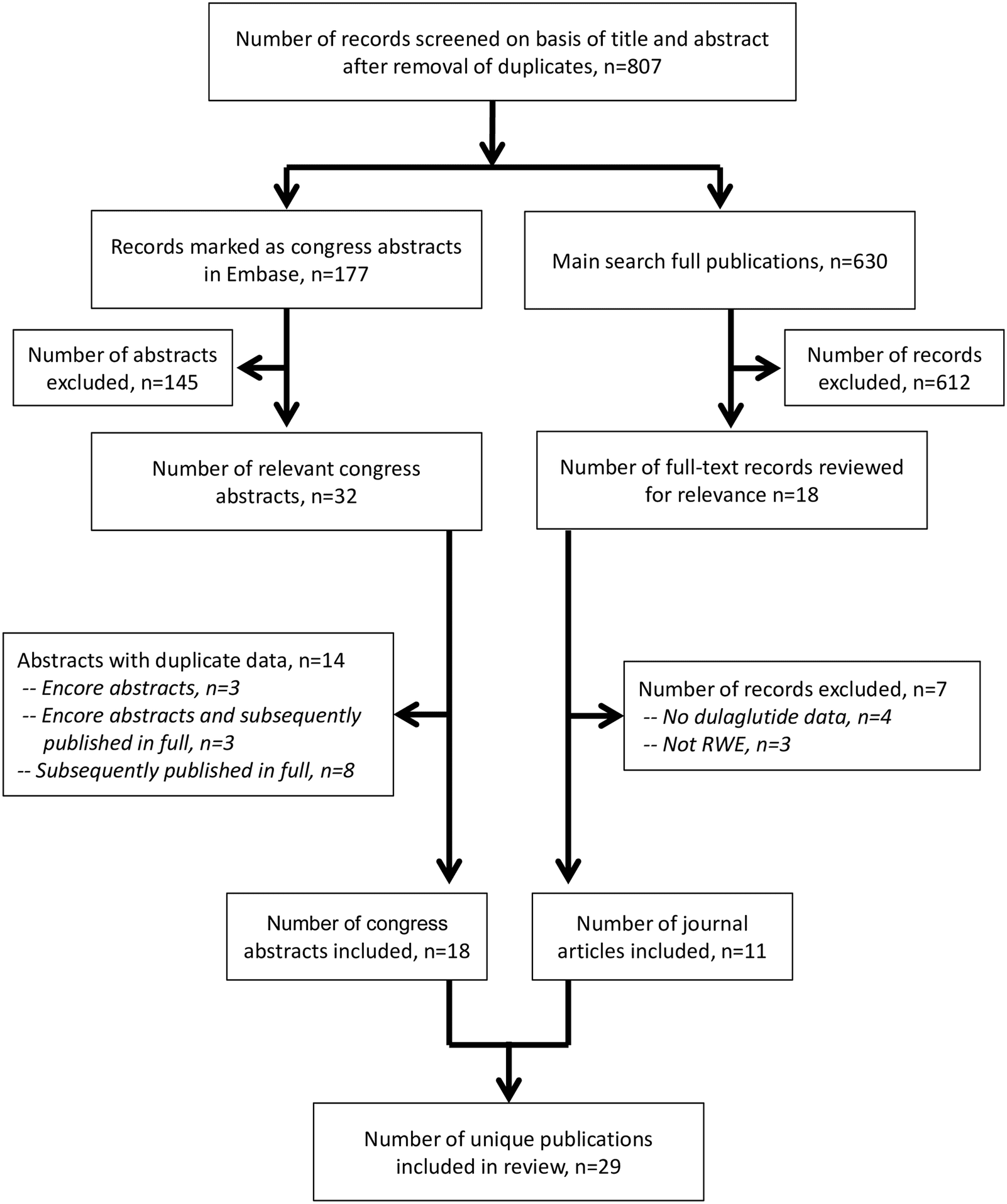 Further corroborating these findings are retrospective studies conducted across various regions, including the EU, Canada, Germany, and the US. In these studies, dulaglutide consistently showed higher rates of treatment adherence and persistence and lower rates of discontinuation compared to other GLP1 RAs, such as liraglutide, exenatide once daily, exenatide twice daily, and lixisenatide. For instance, in a comprehensive analysis of pharmacy claims databases from six countries, dulaglutide recipients exhibited higher persistence rates at the one-year mark compared to recipients of other GLP1 RAs. The mean average daily or weekly doses of dulaglutide aligned with recommended dosages, further highlighting its favorable profile in real-world settings.
These findings underscore the robust clinical benefits and superior real-world outcomes associated with dulaglutide therapy in patients with T2D. The consistently higher rates of treatment adherence, persistence, and glycemic control compared to other GLP1 RAs position dulaglutide as a preferred choice for initiating injectable AHA therapy in this patient population.
Further corroborating these findings are retrospective studies conducted across various regions, including the EU, Canada, Germany, and the US. In these studies, dulaglutide consistently showed higher rates of treatment adherence and persistence and lower rates of discontinuation compared to other GLP1 RAs, such as liraglutide, exenatide once daily, exenatide twice daily, and lixisenatide. For instance, in a comprehensive analysis of pharmacy claims databases from six countries, dulaglutide recipients exhibited higher persistence rates at the one-year mark compared to recipients of other GLP1 RAs. The mean average daily or weekly doses of dulaglutide aligned with recommended dosages, further highlighting its favorable profile in real-world settings.
These findings underscore the robust clinical benefits and superior real-world outcomes associated with dulaglutide therapy in patients with T2D. The consistently higher rates of treatment adherence, persistence, and glycemic control compared to other GLP1 RAs position dulaglutide as a preferred choice for initiating injectable AHA therapy in this patient population.
REWIND Cardiovascular Outcomes Trial
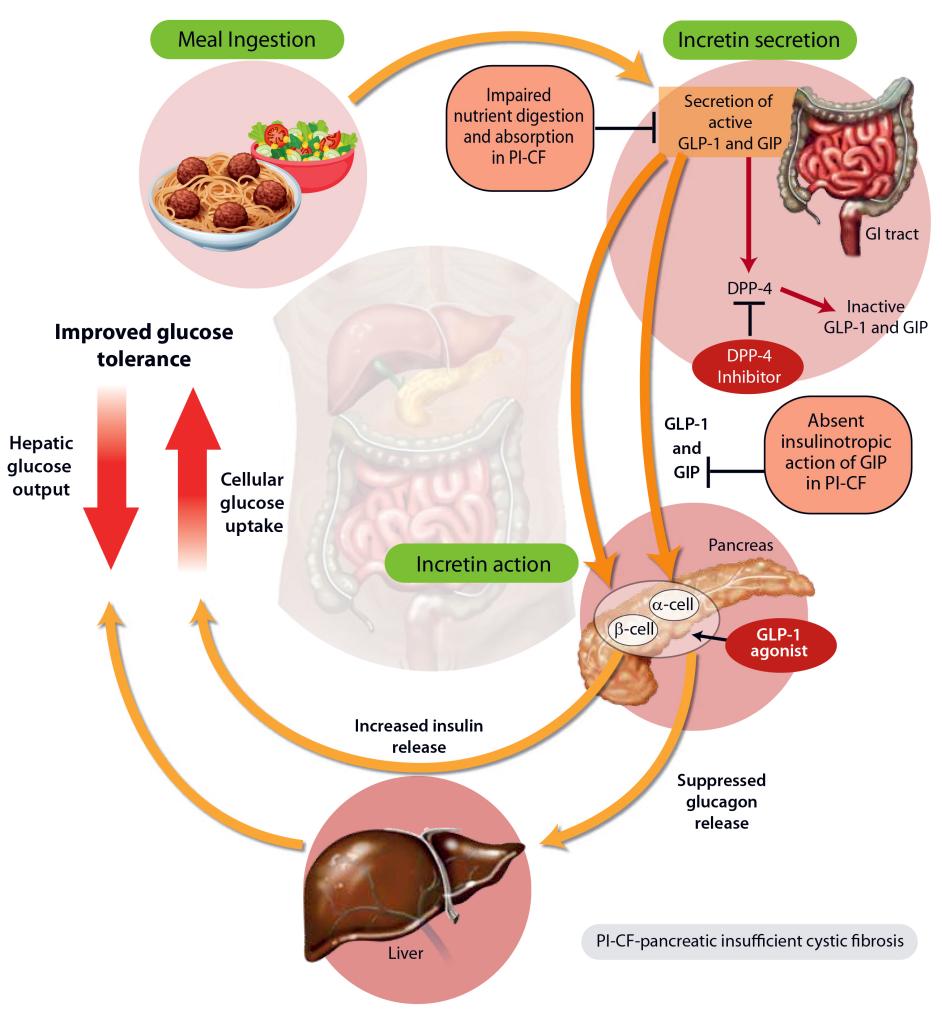
The REWIND cardiovascular outcomes trial (CVOT) was a randomized, double-blind study conducted globally to assess the impact of once-weekly dulaglutide 1.5 mg when added to existing antihyperglycemic agents (AHAs), including oral antidiabetic drugs (OADs) with or without basal insulin. The study enrolled patients aged 50–54 years with previous cardiovascular disease (CVD), aged 55–59 years with either previous CVD or evidence of other renal or vascular disease, or aged 60 years and above with these criteria or two or more cardiovascular risk factors. Participants had a mean age of 66 years, a mean reported disease duration of 10 years, and a mean HbA1c of 7.3% at baseline. The primary composite cardiovascular outcome consisted of cardiovascular death, nonfatal myocardial infarction (MI), or nonfatal stroke. At a median follow-up of 5.4 years, dulaglutide demonstrated a significant 12% reduction in the risk of the primary composite cardiovascular outcome compared to placebo. Subgroup analyses showed consistent benefits across various parameters, including age, gender, and baseline HbA1c. Additionally, dulaglutide showed favorable effects on microvascular outcomes, including renal and eye outcomes. Post hoc exploratory analyses indicated a reduced risk of new macroalbuminuria in the dulaglutide group. Changes in other parameters, such as glycemic control, body weight, blood pressure, and lipid profile, favored dulaglutide over placebo. Safety analyses, supported by a meta-analysis of prospectively adjudicated cardiovascular events from previous dulaglutide trials, revealed no increase in the risk of major adverse cardiovascular events (MACE) with dulaglutide compared to comparators. These findings underscore the cardiovascular and overall metabolic benefits of dulaglutide therapy in patients with type 2 diabetes, providing valuable insights into its efficacy and safety profile in real-world settings.Tolerability of Dulaglutide
Dulaglutide, whether administered as monotherapy or in combination with other antihyperglycemic agents (AHAs), demonstrated generally favorable tolerability profiles in both clinical trial and real-world settings. In pooled analyses of placebo-controlled trials with a mean duration of dulaglutide exposure of 23.8 weeks, common adverse reactions (ARs) reported in ≥5% of dulaglutide recipients included nausea, diarrhea, vomiting, abdominal pain, decreased appetite, dyspepsia, and fatigue, with higher frequencies observed compared to placebo. Notably, gastrointestinal (GI) ARs were the most prevalent, occurring in 31.6%, 41.0%, and 21.3% of patients in the dulaglutide 0.75 mg, dulaglutide 1.5 mg, and placebo groups, respectively. Most GI ARs were of mild or moderate severity, and discontinuations due to GI ARs were infrequent. The incidence of GI ARs peaked during the initial weeks of treatment and subsequently declined. In the REWIND trial, treatment-emergent GI adverse events were more frequent in the dulaglutide group compared to the comparator group, although serious GI adverse events showed no significant difference between the groups. Regarding hypoglycemia, dulaglutide demonstrated low incidence rates when added to various AHA regimens, with generally higher rates observed when combined with sulfonylureas or basal insulin. Sinus tachycardia adverse reactions were reported in dulaglutide recipients, along with first-degree atrioventricular block and increases in PR interval, although the clinical significance of these findings remains unclear. Pancreatitis-related adverse reactions were rare but slightly elevated in dulaglutide recipients compared to non-incretin comparators. The integrated analysis of clinical trials also indicated a slight increase in lipase and/or pancreatic amylase levels in dulaglutide recipients compared to placebo. Additionally, dulaglutide did not show significant differences in the incidence of prespecified adverse events of special interest, including serious renal or urinary events, severe hypoglycemia, acute pancreatitis, serious hepatic events, and thyroid-related adverse events, compared to comparators in the REWIND trial. Overall, dulaglutide exhibited a tolerable safety profile in clinical trials, with GI adverse events being the most commonly reported. While hypoglycemia rates were generally low, they varied depending on the concomitant AHA regimen. Other adverse events of special interest, such as cardiac and pancreatic events, were infrequent but warrant continued monitoring in clinical practice. These findings contribute to a comprehensive understanding of the safety profile of dulaglutide in the management of type 2 diabetes.Place of Dulaglutide in the Management of Type 2 Diabetes
Current international, UK, and US guidelines for type 2 diabetes (T2D) underscore the importance of personalized pharmacotherapy tailored to individual clinical needs and patient preferences. Factors influencing treatment selection include disease manifestations, comorbidities (e.g., obesity, renal disease, cardiovascular disease), patient characteristics (e.g., age, frailty), and drug properties (e.g., cost, route/frequency of administration, safety profile, drug-drug interactions). While specific guidelines may vary slightly in their recommendations regarding the use of particular antihyperglycemic agents (AHAs), metformin is generally recommended as first-line therapy due to its established efficacy, safety, and cost-effectiveness. However, in patients intolerant to or for whom metformin is contraindicated, alternative monotherapy options include sodium-glucose cotransporter 2 inhibitors (SGLT2i), glucagon-like peptide 1 receptor agonists (GLP1 RAs), dipeptidyl peptidase-4 inhibitors, thiazolidinediones, alpha-glucosidase inhibitors, and sulfonylureas. Most patients eventually require intensification of treatment with dual or triple therapy to achieve and maintain glycemic control. Effective management of common comorbidities such as hypertension, obesity, dyslipidemia, and chronic kidney disease (CKD) is essential for reducing overall cardiovascular risks in patients with T2D. Numerous studies have demonstrated that good glycemic control is associated with sustained reductions in the onset and progression of microvascular complications. Large cardiovascular outcome trials (CVOTs) have shown that certain GLP1 RAs and SGLT2i are associated with significant reductions in major adverse cardiovascular events (MACE) and improved renal outcomes in patients with T2D and established atherosclerotic cardiovascular disease (ASCVD) or at high risk of ASCVD. GLP1 RAs and SGLT2i are recommended as first-line therapy for patients with ASCVD or CKD, while SGLT2i are particularly recommended for patients with heart failure (HF) or CKD due to their proven benefits in reducing HF and/or CKD progression. The choice of GLP1 RA or SGLT2i may also be influenced by factors such as device preference, patient adherence, and persistence. Studies have shown that patients often prefer the once-weekly dulaglutide injection pen over other GLP1 RA injection pens, citing ease-of-use and convenience as key factors. Real-world data further support the favorable adherence and persistence rates associated with dulaglutide treatment, possibly due to its single-use device design. Extensive evidence from randomized controlled trials (RCTs) and real-world studies has confirmed the glycemic efficacy and beneficial effects of once-weekly dulaglutide in patients with T2D, including those with high cardiovascular risk or comorbidities such as CKD. Dulaglutide, whether used as monotherapy or in combination with other AHAs, has consistently demonstrated durable glycemic control, weight loss benefits, and improvements in patient-reported outcomes related to diabetes satisfaction and perceived frequency of hyper- and hypoglycemia. Overall, dulaglutide is well tolerated, with gastrointestinal disorders being the most common adverse reactions. Hypoglycemia rates are generally low, but may increase with concomitant use of other hypoglycemic agents. While rare cases of pancreatitis have been reported with GLP1 RA use, the overall incidence of adverse events with dulaglutide remains low. In conclusion, once-weekly dulaglutide stands as an effective and convenient option for the management of T2D, offering sustained glycemic control, cardiovascular benefits, and favorable tolerability in a broad range of patients.Reference
- Scott, L. J. (2020). Dulaglutide: a review in type 2 diabetes. Drugs, 80(2), 197-208.
- Gerstein, H. C., Colhoun, H. M., Dagenais, G. R., Diaz, R., Lakshmanan, M., Pais, P., … & Zucchiatti, N. (2019). Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. The Lancet, 394(10193), 121-130.
- Ludvik, B., Frías, J. P., Tinahones, F. J., Wainstein, J., Jiang, H., Robertson, K. E., … & Milicevic, Z. (2018). Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. The lancet Diabetes & endocrinology, 6(5), 370-381.
- Garber, A. J., Handelsman, Y., Grunberger, G., Einhorn, D., Abrahamson, M. J., Barzilay, J. I., … & Umpierrez, G. E. (2020). Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2020 executive summary. Endocrine Practice, 26(1), 107-139.