Erdafitinib: A Novel Therapy for Advanced Urothelial Carcinoma
Abstract
Erdafitinib, a tyrosine kinase inhibitor, shows promise in treating advanced urothelial carcinoma (UC). It targets FGFRs, frequently altered in UC, inhibiting cell proliferation. Clinical trials reveal its efficacy in FGFR-altered UC, even post-progression on prior therapies. Common adverse events include hyperphosphatemia, stomatitis, fatigue, and ocular toxicities. Research focuses on optimizing erdafitinib use, exploring combination therapies, and biomarker-driven strategies. Erdafitinib is a valuable addition to advanced UC treatments, offering new hope.
Introduction
Urothelial carcinoma (UC) is the most common type of bladder cancer, and it can also affect the renal pelvis, ureter, and urethra. Despite advances in treatment, the prognosis for patients with advanced or metastatic UC remains poor, highlighting the need for new therapeutic approaches. Fibroblast growth factor receptors (FGFRs) are a family of receptor tyrosine kinases that play a key role in cell proliferation, survival, and angiogenesis. Aberrant FGFR signaling, often due to genetic alterations such as gene amplification, mutations, or fusions, has been implicated in the pathogenesis of UC, making FGFRs attractive targets for therapy.
Erdafitinib is a potent and selective oral inhibitor of FGFR1-4, with a higher affinity for FGFR2 and FGFR3. By inhibiting FGFR signaling, erdafitinib blocks downstream pathways involved in cell growth and survival, leading to antitumor effects. Erdafitinib received accelerated approval from the United States Food and Drug Administration (FDA) in 2019 for the treatment of locally advanced or metastatic UC with susceptible FGFR2 or FGFR3 genetic alterations that have progressed during or following platinum-containing chemotherapy. This approval was based on the results of the Phase 2 BLC2001 trial, which demonstrated significant clinical benefit with erdafitinib in this patient population.
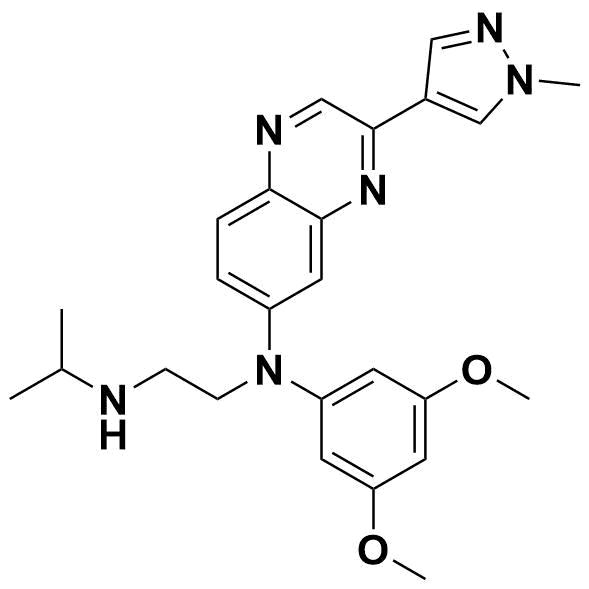
Structural formula of Erdafitinib
Mechanism of Action
FGFRs are transmembrane receptor tyrosine kinases that consist of an extracellular ligand-binding domain, a transmembrane domain, and an intracellular tyrosine kinase domain. Upon binding of fibroblast growth factors (FGFs) to the extracellular domain, FGFRs dimerize and undergo autophosphorylation, leading to activation of downstream signaling pathways such as the RAS-MAPK and PI3K-AKT pathways. Aberrant FGFR signaling, resulting from genetic alterations in FGFRs or FGFs, can drive uncontrolled cell proliferation and survival, contributing to tumorigenesis.
Erdafitinib exerts its antitumor effects by selectively inhibiting FGFR1-4, thereby blocking FGFR signaling. In preclinical studies, erdafitinib has been shown to inhibit proliferation and induce apoptosis in cancer cell lines with FGFR alterations. Erdafitinib has also demonstrated antitumor activity in FGFR-altered xenograft models. Clinical studies have confirmed the activity of erdafitinib in patients with FGFR-altered UC, supporting its mechanism of action in this setting.
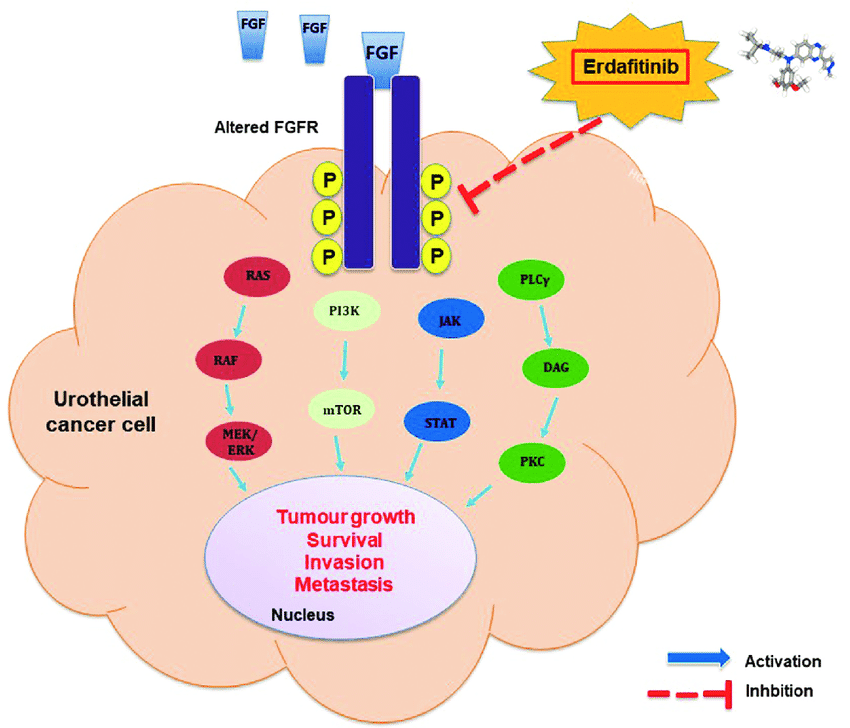
FGFR signalling pathway and the molecular mechanisms of erdafitinib in urothelial carcinoma
Pharmacokinetics
Erdafitinib is administered orally, with recommended dosing of 8 mg once daily. It is rapidly absorbed, with peak plasma concentrations reached within 2 hours of dosing. Erdafitinib exhibits dose-proportional pharmacokinetics over a dose range of 0.5-12 mg. Steady-state concentrations are typically achieved within 15 days of daily dosing. Erdafitinib is extensively metabolized in the liver, primarily by CYP2C9 and to a lesser extent by CYP3A4. The main circulating metabolite, N-desmethyl erdafitinib, is pharmacologically active and contributes to the overall efficacy of erdafitinib. Erdafitinib and its metabolites are primarily eliminated in the feces, with minimal renal excretion.
Clinical Efficacy
The clinical efficacy of erdafitinib has been evaluated in several clinical trials, including the Phase 2 BLC2001 trial. In this study, patients with locally advanced or metastatic UC with susceptible FGFR2 or FGFR3 genetic alterations who had progressed on prior platinum-containing chemotherapy were treated with erdafitinib. The primary endpoint was the objective response rate (ORR) assessed by an independent review. Secondary endpoints included duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety.
The BLC2001 trial demonstrated a clinically meaningful ORR of 40.6% (95% CI: 31.8-49.9) with erdafitinib, including a complete response rate of 3.4% and a partial response rate of 37.2%. The median DOR was 5.6 months (95% CI: 4.2-6.9), and the median PFS was 5.5 months (95% CI: 4.2-6.9). The median OS was 13.8 months (95% CI: 10.4-16.7). Subgroup analyses showed consistent efficacy across various patient subgroups, including those with different FGFR alterations and prior lines of therapy.
Safety Profile
The safety profile of erdafitinib was consistent with that observed in previous studies. The most common adverse events (AEs) associated with erdafitinib included hyperphosphatemia, stomatitis, fatigue, and ocular toxicities (e.g., dry eye, blurred vision). Grade 3-4 AEs occurred in 52% of patients, with the most common being hyperphosphatemia (17%) and stomatitis (7%). Dose reductions or interruptions due to AEs were required in 46% of patients, highlighting the importance of proactive management of AEs with erdafitinib therapy.
Future Directions
Ongoing research aims to further optimize the use of erdafitinib in the treatment of UC. Combination therapies, including immunotherapy and chemotherapy, are being investigated to enhance the antitumor effects of erdafitinib. Biomarker-driven treatment strategies are also being explored to identify patients most likely to benefit from erdafitinib based on their tumor molecular profile. Additionally, efforts are underway to elucidate mechanisms of resistance to erdafitinib and develop strategies to overcome them.
Conclusion
Erdafitinib is a novel therapy that has demonstrated significant clinical benefit in patients with advanced or metastatic UC with FGFR2 or FGFR3 genetic alterations who have progressed on prior platinum-containing chemotherapy. Its mechanism of action, pharmacokinetics, clinical efficacy, and safety profile support its use as a valuable treatment option for this challenging disease. Ongoing research is focused on optimizing the use of erdafitinib through combination therapies and biomarker-driven approaches, to improve outcomes for patients with UC. As we continue to expand our understanding of the molecular drivers of UC and the mechanisms of action of erdafitinib, we are hopeful that erdafitinib will continue to play a vital role in the treatment landscape of advanced UC. Collaborative efforts between researchers, clinicians, and patients are essential to furthering our knowledge and translating it into improved patient care. Erdafitinib represents a significant advancement in the field of UC therapy and underscores the importance of personalized medicine in oncology.
In conclusion, erdafitinib is a promising therapy for patients with advanced or metastatic UC with FGFR2 or FGFR3 genetic alterations. Its selective inhibition of FGFR signaling, coupled with its manageable safety profile and demonstrated clinical efficacy, make it a valuable addition to the treatment armamentarium for UC. Ongoing research is focused on optimizing its use and identifying strategies to overcome resistance, with the ultimate goal of improving outcomes for patients with this challenging disease.
References
- Franza A, Pirovano M, Giannatempo P, Cosmai L. Erdafitinib in locally advanced/metastatic urothelial carcinoma with certain FGFR genetic alterations. Future Oncol. 2022 Jun;18(19):2455-2464. doi: 10.2217/fon-2021-1151. Epub 2022 Apr 7. PMID: 35387485.
- Loriot Y, O’Hagan A, Siefker-Radtke AO. Plain language summary of erdafitinib in locally advanced or metastatic urothelial carcinoma: a phase 2 study with long-term follow-up. Future Oncol. 2023 Nov 2. doi: 10.2217/fon-2023-0596. Epub ahead of print. PMID: 37916514.
- Ascione CM, Napolitano F, Esposito D, Servetto A, Belli S, Santaniello A, Scagliarini S, Crocetto F, Bianco R, Formisano L. Role of FGFR3 in bladder cancer: Treatment landscape and future challenges. Cancer Treat Rev. 2023 Apr;115:102530. doi: 10.1016/j.ctrv.2023.102530. Epub 2023 Feb 28. PMID: 36898352.
- Benjamin DJ, Mar N, Rezazadeh Kalebasty A. Immunotherapy With Checkpoint Inhibitors in FGFR-Altered Urothelial Carcinoma. Clin Med Insights Oncol. 2022 Sep 27;16:11795549221126252. doi: 10.1177/11795549221126252. PMID: 36186672; PMCID: PMC9520173.
- Iacovelli R, Cicala CM, Ciccarese C, Sacco E, Racioppi M, Bassi PF, Tortora G. Management of metastatic urothelial carcinoma: Current approach, emerging agents, and future perspectives. Urologia. 2023 Feb;90(1):3-10. doi: 10.1177/03915603221139907. Epub 2022 Dec 20. PMID: 36537831.
- Peng J, Sridhar S, Siefker-Radtke AO, Selvarajah S, Jiang DM. Targeting the FGFR Pathway in Urothelial Carcinoma: the Future Is Now. Curr Treat Options Oncol. 2022 Sep;23(9):1269-1287. doi: 10.1007/s11864-022-01009-4. Epub 2022 Aug 13. PMID: 35962938.
- Wekking D, Pretta A, Martella S, D’Agata AP, Joeun Choe J, Denaro N, Solinas C, Scartozzi M. Fibroblast growth factor receptors as targets for anticancer therapy in cholangiocarcinomas and urothelial carcinomas. Heliyon. 2023 Aug 27;9(9):e19541. doi: 10.1016/j.heliyon.2023.e19541. PMID: 37681152; PMCID: PMC10481293.