Tukysa (Tucatinib): A Novel Treatment for HER2-Positive Breast Cancer
Abstract
Tukysa (tucatinib) is a recently developed medication for the treatment of HER2-positive breast cancer. This paper provides an overview of the mechanism of action, clinical efficacy, safety profile, and future directions of Tukysa in the management of HER2-positive breast cancer. Tukysa, as a tyrosine kinase inhibitor, has shown promising results in clinical trials, particularly in combination with other HER2-targeted therapies, offering new hope for patients with advanced or metastatic HER2-positive breast cancer.
Introduction
Breast cancer encompasses various subtypes, including HER2-positive breast cancer, distinguished by excessive human epidermal growth factor receptor 2 (HER2) protein expression. This subtype often displays heightened aggressiveness and a less favorable prognosis than others. Targeted treatments, like HER2-targeted monoclonal antibodies (e.g., trastuzumab, pertuzumab) and tyrosine kinase inhibitors (e.g., lapatinib, neratinib), have notably enhanced outcomes in HER2-positive breast cancer patients. These therapies work by specifically targeting HER2, hindering its activity and impeding tumor growth. They represent a crucial advancement in managing HER2-positive breast cancer, providing more effective and tailored treatment options for patients.
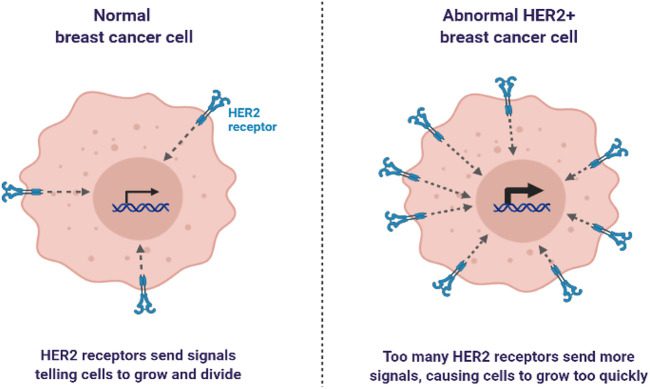
Fig 1. Representation of normal breast cancer cell and abnormal breast cancer cell.
Mechanism of Action
Tukysa, also known as tucatinib, is a small molecule inhibitor designed to target the HER2 tyrosine kinase, a key player in promoting cell proliferation and survival in HER2-positive breast cancer. By binding to the intracellular domain of the HER2 protein, Tukysa effectively blocks its kinase activity, disrupting the signaling pathways responsible for tumor growth. This targeted inhibition of HER2 signaling not only suppresses cell proliferation but also induces apoptosis, or programmed cell death, in HER2-positive cancer cells.
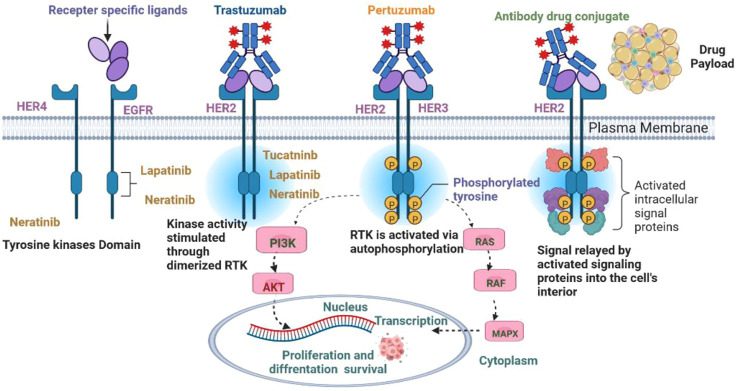
Fig 2. Signaling pathways in HER2+ breast cancer.
The unique mechanism of action of Tukysa distinguishes it from other HER2-targeted therapies, offering a more precise and effective approach to treating HER2-positive breast cancer. Clinical studies have shown that Tukysa when used in combination with other HER2-targeted therapies and chemotherapy, can significantly improve progression-free survival and overall survival rates in patients with HER2-positive metastatic breast cancer. Tukysa represents a promising new treatment option for HER2-positive breast cancer, providing hope for improved outcomes in patients with this aggressive form of the disease.
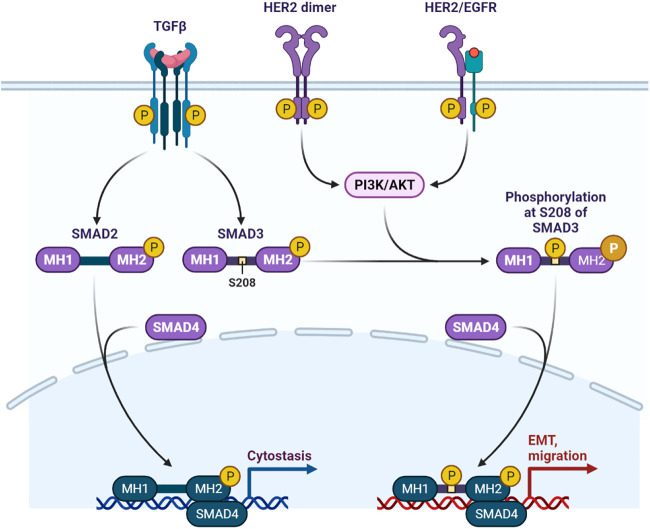
Fig 3. HER2/EGFR signaling pathway in breast cancer.
Clinical Efficacy
Clinical trials have confirmed the effectiveness of Tukysa in treating HER2-positive breast cancer. In the HER2CLIMB trial, a phase 2 study, researchers investigated Tukysa in combination with trastuzumab and capecitabine in patients with HER2-positive metastatic breast cancer who had previously been treated with trastuzumab, pertuzumab, and ado-trastuzumab emtansine (T-DM1). The results demonstrated a significant improvement in both progression-free survival (PFS) and overall survival (OS) compared to a placebo in combination with trastuzumab and capecitabine. These findings highlight the efficacy of Tukysa as an important addition to the treatment options for HER2-positive breast cancer, particularly in cases where standard treatments have not been effective.
Safety Profile
Tukysa is generally well-tolerated, with a manageable safety profile. Among the most frequently reported adverse reactions are diarrhea, nausea, fatigue, elevated liver enzymes, vomiting, and decreased appetite. Diarrhea, in particular, can be severe in certain instances. However, with careful management, such as dose adjustments and the use of anti-diarrheal medications, most patients can continue their treatment with Tukysa.
Effective management of diarrhea is crucial for patients receiving Tukysa. Strategies to mitigate this side effect include the prompt initiation of anti-diarrheal medications, dose modifications based on the severity of symptoms, and close monitoring by healthcare providers. These measures not only help alleviate diarrhea but also enable patients to maintain their treatment regimen, thereby maximizing the potential benefits of Tukysa therapy.
Overall, while Tukysa may be associated with some adverse effects, these can be effectively managed with appropriate interventions, allowing patients to continue their treatment and potentially improve their outcomes in HER2-positive breast cancer.
Future Directions
Tukysa, or tucatinib, stands as a significant advancement in the treatment landscape for HER2-positive breast cancer. Its novel mechanism of action and promising clinical results have sparked interest in further optimizing its use. Future research should prioritize elucidating the optimal sequencing and combination strategies involving Tukysa and other HER2-targeted therapies. Understanding the most effective ways to integrate Tukysa into treatment regimens could enhance its efficacy and improve patient outcomes.
Moreover, ongoing studies are exploring the potential of Tukysa in earlier stages of HER2-positive breast cancer. Investigating its role in neoadjuvant or adjuvant settings could provide valuable insights into its ability to improve outcomes in earlier stages of the disease. Additionally, research is underway to evaluate Tukysa’s efficacy in other HER2-positive malignancies, such as HER2-positive gastric cancer. These studies aim to expand the therapeutic options available for patients with these challenging diseases and potentially improve their prognosis.
In conclusion, Tukysa represents a significant step forward in the treatment of HER2-positive breast cancer. Continued research into its optimal use, both in terms of sequencing and combination strategies, could further enhance its clinical benefit. Additionally, exploring its role in earlier stages of HER2-positive breast cancer and other HER2-positive malignancies could broaden its impact and offer new hope to patients with these conditions.
Conclusion
Tukysa holds promise as a valuable addition to the treatment options available for HER2-positive breast cancer. Its distinctive mechanism of action, targeting the HER2 tyrosine kinase, sets it apart from other therapies. This specificity, coupled with its favorable clinical efficacy and manageable safety profile, positions Tukysa as a valuable choice for patients with advanced or metastatic HER2-positive breast cancer.
Ongoing research is vital to further understanding Tukysa’s optimal use in managing HER2-positive breast cancer and other related malignancies. This includes exploring its potential in earlier stages of HER2-positive breast cancer and its efficacy in combination with other HER2-targeted therapies. Such investigations aim to maximize the benefits of Tukysa while minimizing potential side effects, ultimately improving outcomes for patients with HER2-positive breast cancer. As research continues to evolve, Tukysa’s role in the treatment landscape is likely to become more defined, offering new hope for patients facing these challenging diseases.
References
- Mercogliano MF, Bruni S, Mauro FL, Schillaci R. Emerging Targeted Therapies for HER2-Positive Breast Cancer. Cancers (Basel). 2023 Mar 26;15(7):1987. doi: 10.3390/cancers15071987. PMID: 37046648; PMCID: PMC10093019.
- Barot SV, Roesch E, Abraham J. Optimizing adjuvant and post-neoadjuvant therapy in HER2-positive early breast cancer. Expert Rev Anticancer Ther. 2022 Dec;22(12):1289-1299. doi: 10.1080/14737140.2022.2146580. Epub 2022 Nov 17. PMID: 36373394.
- Stanowicka-Grada M, Senkus E. Anti-HER2 Drugs for the Treatment of Advanced HER2 Positive Breast Cancer. Curr Treat Options Oncol. 2023 Nov;24(11):1633-1650. doi: 10.1007/s11864-023-01137-5. Epub 2023 Oct 25. PMID: 37878202; PMCID: PMC10643304.
- Singh DD, Lee HJ, Yadav DK. Clinical updates on tyrosine kinase inhibitors in HER2-positive breast cancer. Front Pharmacol. 2022 Dec 12;13:1089066. doi: 10.3389/fphar.2022.1089066. PMID: 36578543; PMCID: PMC9792097.
- Suppan C, Balic M. Current Standards and Future Outlooks in Metastatic Her2-Positive Breast Cancer. Breast Care (Basel). 2023 Feb;18(1):69-75. doi: 10.1159/000528756. Epub 2022 Dec 20. PMID: 36876168; PMCID: PMC9982349.
- Sirhan Z, Thyagarajan A, Sahu RP. The efficacy of tucatinib-based therapeutic approaches for HER2-positive breast cancer. Mil Med Res. 2022 Jul 13;9(1):39. doi: 10.1186/s40779-022-00401-3. PMID: 35820970; PMCID: PMC9277867.
- Bansal I, Pandey AK, Ruwali M. Small-molecule inhibitors of kinases in breast cancer therapy: recent advances, opportunities, and challenges. Front Pharmacol. 2023 Aug 30;14:1244597. doi: 10.3389/fphar.2023.1244597. PMID: 37711177; PMCID: PMC10498465.
- Ferrando-Díez A, Felip E, Pous A, Bergamino Sirven M, Margelí M. Targeted Therapeutic Options and Future Perspectives for HER2-Positive Breast Cancer. Cancers (Basel). 2022 Jul 6;14(14):3305. doi: 10.3390/cancers14143305. PMID: 35884366; PMCID: PMC9320771.