Folate Receptor: A New Horizon in Cancer Therapeutics
Abstract
In the vast landscape of oncological research, identifying precise targets for therapy stands at the forefront of innovation. Among such targets, the folate receptor (FR), especially its alpha variant (FRα), presents an exceptional avenue for the development of targeted cancer treatments. Folate, a B vitamin crucial for DNA synthesis and repair, plays an integral role in cell division and growth. The pathologies of cancer often exploit these basic cellular functions for malignant progression, making pathways involving nutrients like folate critical focal points for therapeutic intervention.
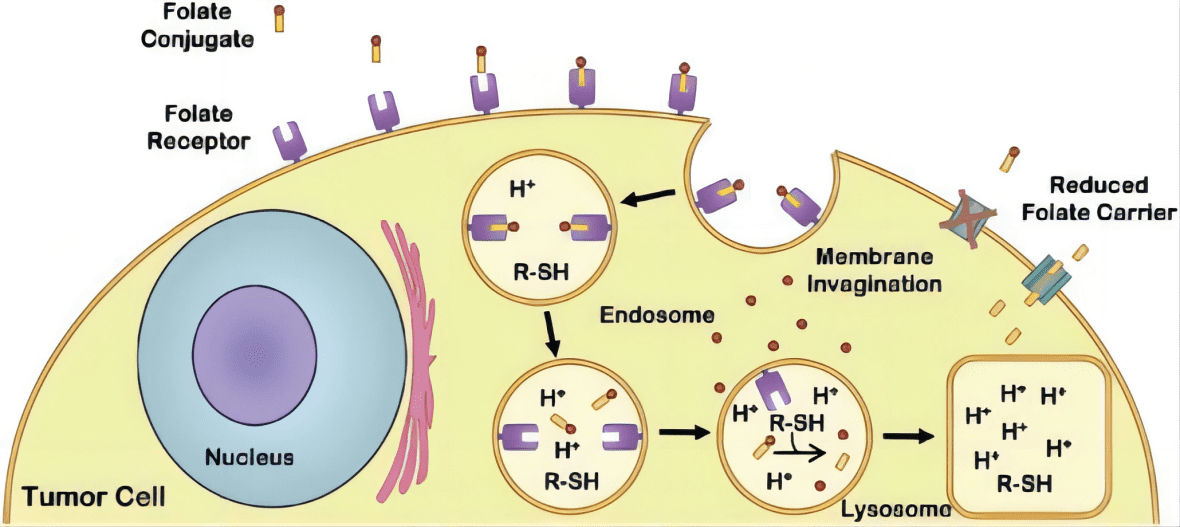
Folate’s importance extends beyond basic nutrition; it is vital for the creation and repair of DNA, processes that are hijacked by cancer cells to foster rapid, uncontrolled growth. The enzyme systems involved in folate metabolism are often upregulated in cancers, reflecting the increased demand for DNA synthesis materials in rapidly dividing cells. The body’s mechanism to procure folate involves a specific membrane-bound protein known as the folate receptor, which exists in several isoforms, with FRα being the most prominently linked to cancer.
The Role of the Folate Receptor in Oncology
FRα’s overexpression in cancerous tissues, as opposed to normal tissues, makes it a prime target for diagnostic and therapeutic strategies. Elevated levels of FRα are particularly notable in cancers of the breast, ovary, and lung, where it facilitates the increased uptake of folate necessary for the rapid cell proliferation characteristic of tumors. This differential expression offers a pathway to not only hone in on cancer cells with greater precision during treatment but also to monitor disease progression and response to therapies.
Development of Folate-Based Diagnostic and Therapeutic Tools
The distinct presence of FRα in tumors underpins the development of diagnostic tools that can detect its expression, helping to guide the prognosis and treatment choices. Beyond diagnostics, the therapeutic implications of targeting FRα are profound. Strategies include crafting drugs that specifically bind to FRα, effectively targeting the cancer cells while sparing normal cells, thereby minimizing the typical side effects associated with chemotherapy.
Targeted Therapeutic Strategies: Harnessing FRα
Folate-Drug Conjugates
One of the most promising approaches in exploiting FRα involves folate-drug conjugates. These are sophisticated formulations where a chemotherapy agent is directly linked to a folate molecule. This design leverages the high affinity of FRα for folate, ensuring that the drug is preferentially taken up by cancer cells overexpressing the receptor. Upon binding to FRα, these conjugates are internalized by the cancer cells through endocytosis, releasing the cytotoxic agent intracellularly. This method significantly enhances the drug’s efficacy at the tumor site and reduces systemic exposure, thereby limiting adverse effects.
Antibody-Based Therapies
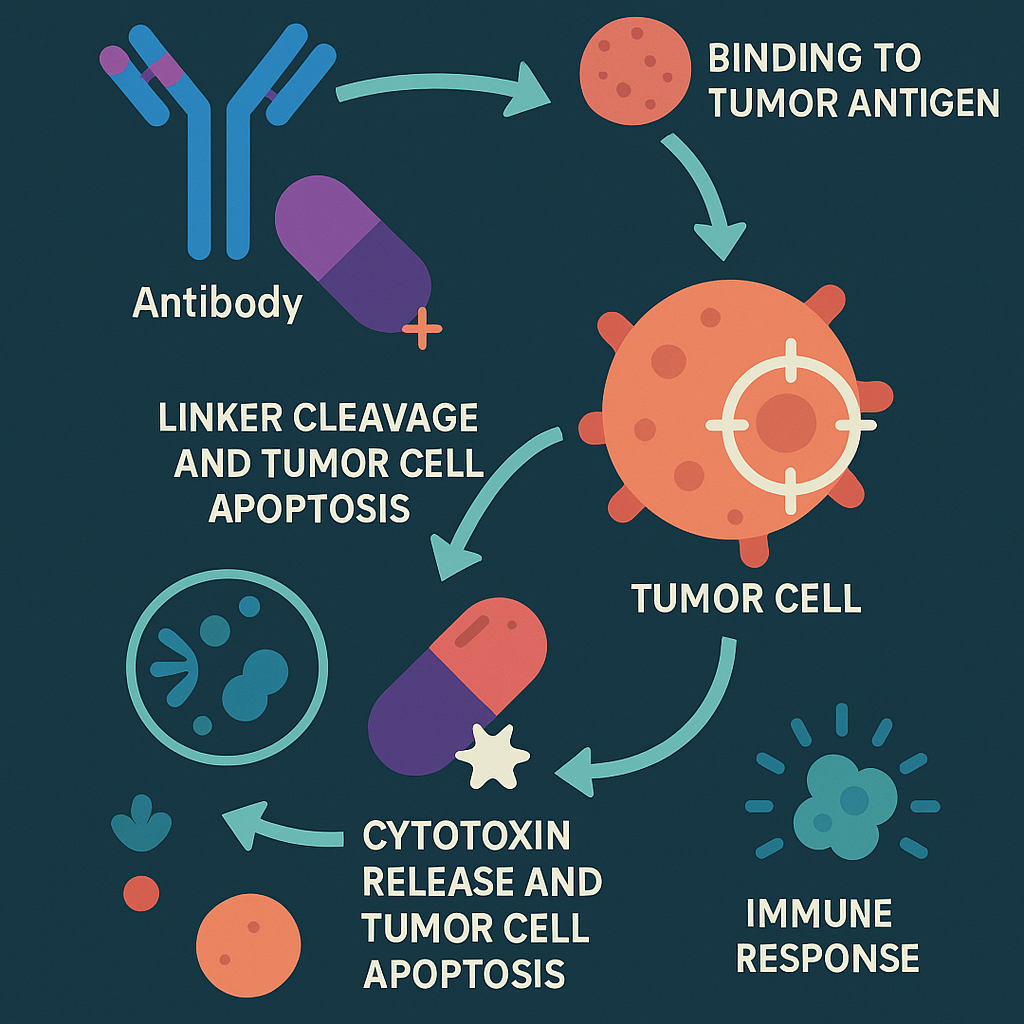
Another innovative approach is the development of monoclonal antibodies that target FRα. These antibodies can be engineered to deliver toxic payloads to the cancer cells or to recruit the immune system to target and destroy the cancer cells. Such therapies, known as antibody-drug conjugates (ADCs), have shown potential in providing more precise and potent anticancer activity while sparing normal tissues. These antibodies can also block the interaction of folate with its receptor, starving the cancer cells of a necessary nutrient, which can inhibit tumor growth.
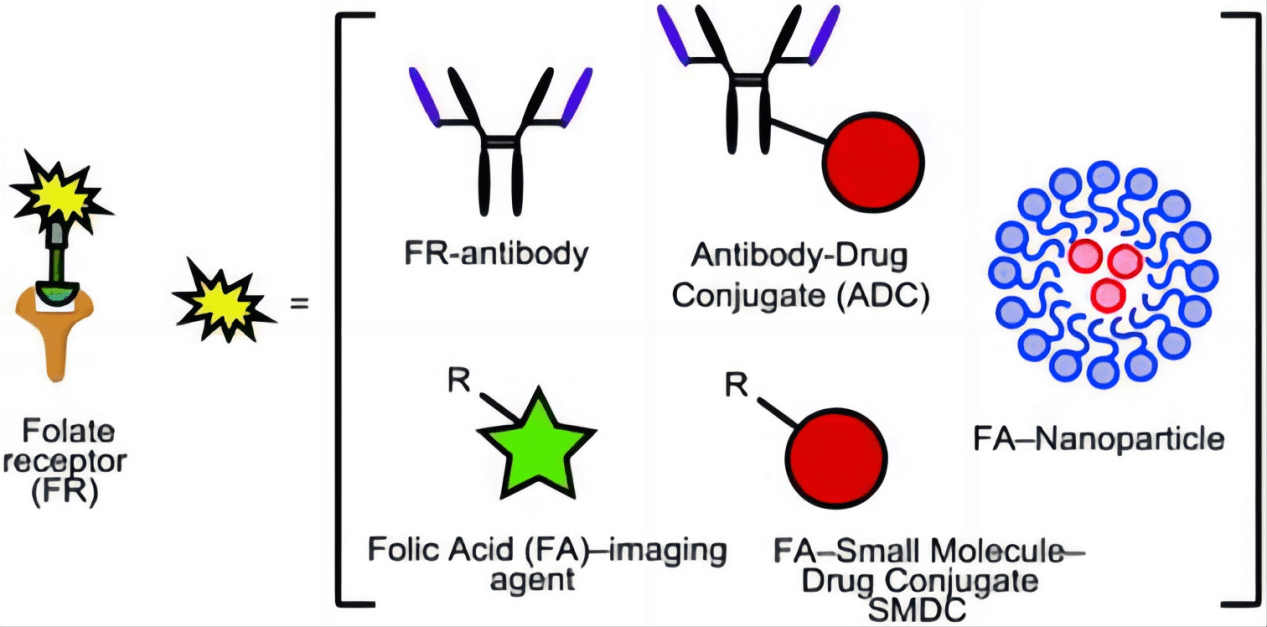
Radioimmunotherapy
Radioimmunotherapy represents a fusion of radioactive therapy and immunotherapy, where radioactive isotopes are attached to antibodies that target FRα. This approach allows for targeted radiation therapy, which delivers lethal radioactive doses directly to the tumor cells, minimizing radiation exposure to healthy tissues.
Clinical Trials and Outcomes
The theoretical advantage of targeting FRα has been supported by a number of clinical trials, testing various folate-based therapies across different types of cancer:
Ovarian Cancer Trials: Vintafolide, a folate-drug conjugate, showed promise in early trials by improving the progression-free survival of patients with ovarian cancer who exhibited high FRα expression. However, subsequent larger trials faced challenges, highlighting the complexities of translating early success into broader clinical practice.
Breast Cancer Research: Trials involving folate conjugates in breast cancer are exploring whether these therapies can provide a viable alternative or complement to traditional treatments, especially in cases resistant to standard therapies.
Non-Small Cell Lung Cancer (NSCLC): Pemetrexed, an antifolate chemotherapeutic, has become a standard treatment for NSCLC, especially in patients with folate receptor expression. Its effectiveness has helped validate the concept of folate pathway targeting in cancer therapy.
Despite the promise, not all trials have yielded positive results, and the effectiveness of FR-targeted therapies often varies significantly between different patient populations and cancer subtypes. This variability underscores the necessity for more personalized approaches in cancer treatment, where the molecular and genetic profiles of tumors could dictate therapeutic strategies.
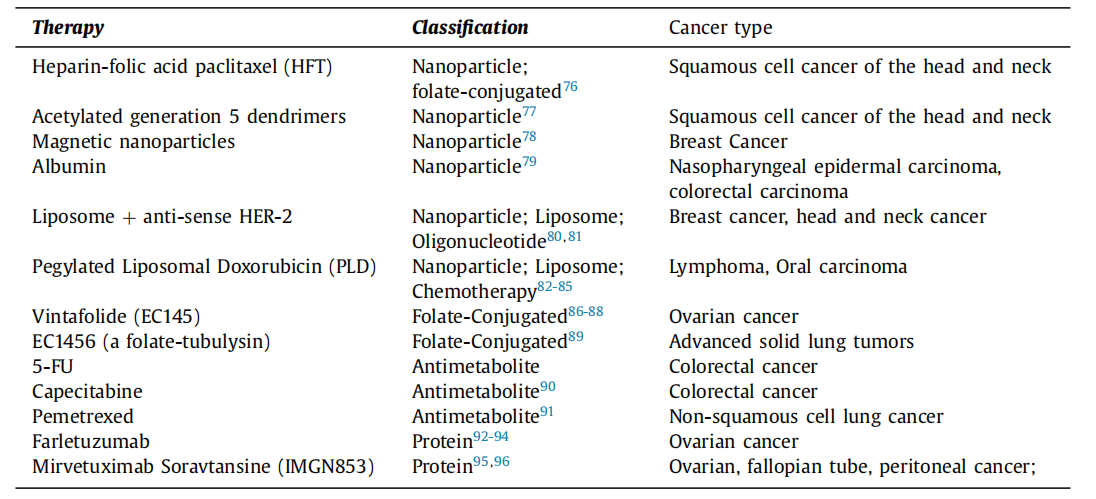
Table 1 Folate-mediated therapeutics and disease states under investigation
Challenges in Folate Receptor-Targeted Therapy
Despite the innovative nature and potential of targeting the folate receptor, several challenges hinder broader application and efficacy:
Heterogeneity of Tumor Expression: The expression of FRα varies widely among different tumors and even within the same tumor type, which can affect the outcome of folate-based therapies. This variation necessitates precise diagnostic tools to identify suitable candidates for these therapies, emphasizing the importance of personalized medicine in oncology.
Development of Resistance: Cancer cells can develop resistance to therapies that target the folate receptor, either through genetic mutations that alter the receptor or through adaptive cellular mechanisms that bypass the blocked pathways. Understanding these resistance mechanisms is crucial for developing second-generation therapies that are more effective and less susceptible to resistance.
Optimization of Drug Delivery: Achieving optimal delivery and efficacy of folate-conjugated drugs and antibodies remains a significant challenge. Enhancements in drug design, such as improving the stability of conjugates and increasing their affinity for FRα, are areas of active research. Additionally, exploring combination therapies that can synergize with folate receptor targeting could improve outcomes.
Future Directions and Potential Impact
The future of folate receptor-targeted therapies looks promising, driven by ongoing research and technological advancements. Here are key areas where significant progress is expected:
Advanced Diagnostics: Developing more sophisticated diagnostic tools to accurately quantify FRα expression and predict therapy responsiveness will be critical. Techniques such as liquid biopsies could provide non-invasive methods to monitor FRα levels and assess tumor dynamics in real-time.
Combination Therapies: Combining folate receptor-targeted therapies with other treatment modalities, such as immune checkpoint inhibitors or traditional chemotherapy, could enhance therapeutic outcomes and prevent or delay the onset of resistance.
Nanotechnology: Leveraging nanotechnology to improve the delivery of folate-conjugated drugs could revolutionize the field. Nanoparticles designed to target FRα can be loaded with multiple therapeutic agents, including drugs and genetic material, to simultaneously attack cancer cells on several fronts.
Genetic Profiling and Personalized Medicine: Integrating comprehensive genetic profiling into clinical practice will help identify patients who will benefit the most from folate receptor-targeted therapies. This approach will tailor treatments based on individual genetic and molecular characteristics, maximizing efficacy and minimizing toxicity.
Conclusion
The exploration of the folate receptor, particularly FRα, as a therapeutic target marks a significant advance in the quest for more precise and effective cancer treatments. The specificity offered by targeting FRα holds the promise of high efficacy coupled with reduced toxicity, embodying the goals of modern cancer therapy. As research progresses, it is anticipated that folate receptor-targeted therapies will become integral to personalized cancer treatment plans, tailored to the unique molecular profiles of each patient’s tumor.
In conclusion, the journey from the laboratory to the clinic is filled with challenges but also abundant with opportunities for innovative treatments that could significantly improve outcomes for cancer patients. The continued exploration and development of folate receptor-targeted therapies not only promise to enhance our therapeutic arsenal but also offer a deeper understanding of cancer biology.
Reference
- Young, O., Ngo, N., Lin, L., Stanbery, L., Creeden, J. F., Hamouda, D., & Nemunaitis, J. (2023). Folate receptor as a biomarker and therapeutic target in solid tumors. Current Problems in Cancer, 47(1), 100917.
- Qin, T., Du, M., Du, H., Shu, Y., Wang, M., & Zhu, L. (2015). Folic acid supplements and colorectal cancer risk: meta-analysis of randomized controlled trials. Scientific reports, 5(1), 1-7.
- Scaranti, M., Cojocaru, E., Banerjee, S., & Banerji, U. (2020). Exploiting the folate receptor α in oncology. Nature reviews clinical oncology, 17(6), 349-359.
- Hussain, A., Kumar, A., Uttam, V., Sharma, U., Sak, K., Saini, R. V., … & Sethi, G. (2023). Application of curcumin nanoformulations to target folic acid receptor in cancer: Recent trends and advances. Environmental Research, 116476.