The Role of Sulfonylureas and Glinides in the Treatment of Type 2 Diabetes
Type 2 Diabetes and Oral Hypoglycemic Drugs
Type 2 diabetes is a chronic metabolic disorder that is characterized by high levels of blood glucose resulting from insulin resistance and/or reduced insulin secretion. It is a major public health problem affecting millions of people worldwide and is associated with a range of serious complications, including cardiovascular disease, kidney disease, and blindness[1].
The mainstay of treatment for type 2 diabetes includes lifestyle modifications such as weight loss, dietary changes, and exercise, along with oral hypoglycemic drugs and/or insulin therapy. Oral hypoglycemic drugs are often used as a first-line therapy to manage blood glucose levels in patients with type 2 diabetes[2]. These drugs act by increasing insulin secretion, decreasing hepatic glucose production, and improving insulin sensitivity.
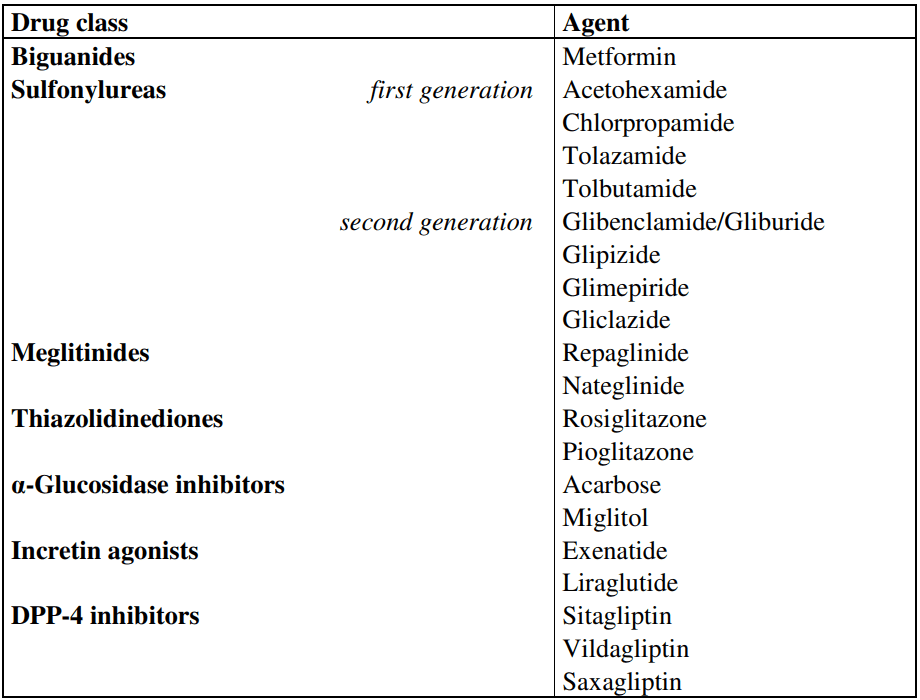
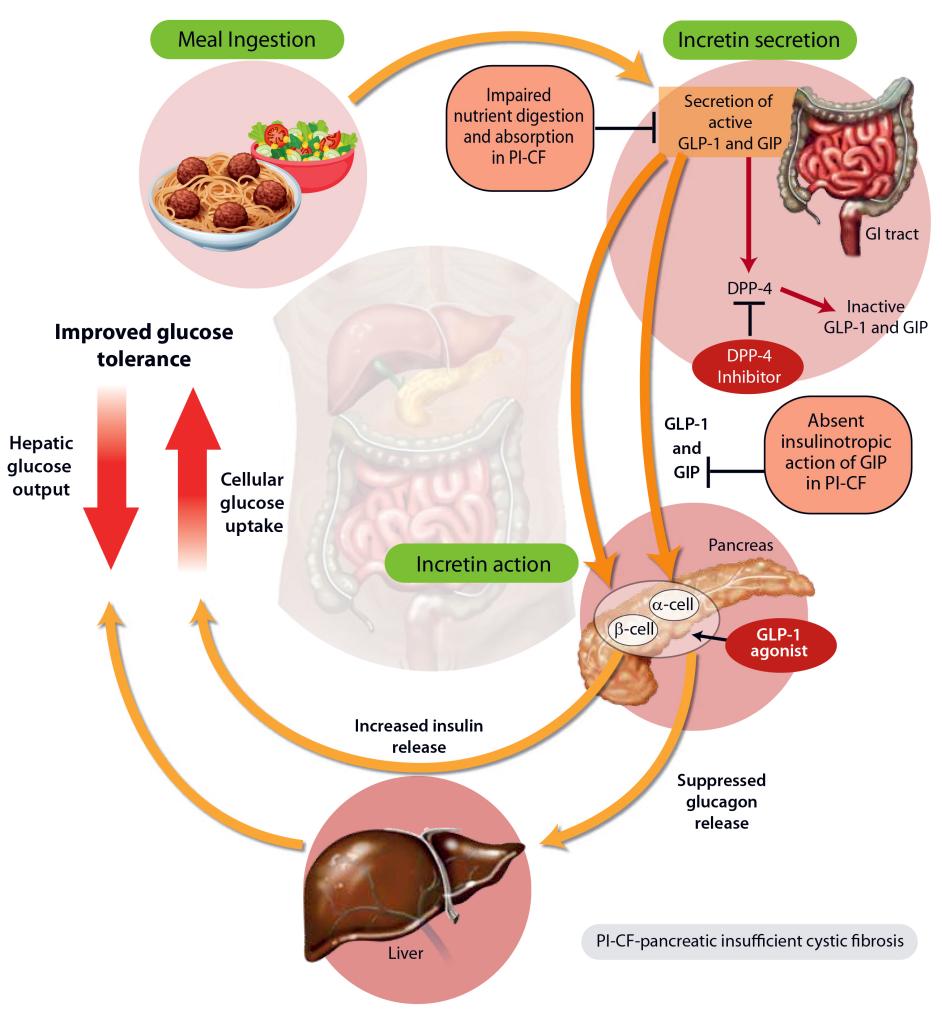
Figure 1 Oral hypoglycemic drugs[2]
There exist multiple categories of oral hypoglycemic medications that include sulfonylureas, biguanides, thiazolidinediones, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium-glucose cotransporter-2 (SGLT2) inhibitors, and glinides. Each classification of drugs has its distinct mechanism of action, pharmacokinetics, and clinical profile. The choice of medication depends on the individual patient’s clinical characteristics and preferences.
Employing oral hypoglycemic drugs is a significant aspect of managing type 2 diabetes as it aids in enhancing glycemic control, decreasing the chances of complications, and improving the patient’s quality of life. Nevertheless, the use of these drugs comes with certain risks, and it is crucial to thoroughly evaluate the potential benefits and risks of each medication before beginning the treatment.
Introduction of Sulfonylureas and Glinides
Sulfonylureas and glinides are two classes of oral hypoglycemic drugs that are commonly used to manage blood glucose levels in patients with type 2 diabetes[3].
For more than half a century, Sulfonylureas have been utilized as the oldest category of oral hypoglycemic medications to regulate blood glucose levels. They function by exciting insulin secretion from the pancreas’s beta cells, thus boosting the availability of insulin to transfer glucose from the bloodstream to cells. Sulfonylureas comprise two generations, with the second generation having a longer duration of impact and a lower likelihood of hypoglycemia when compared to the first generation. Glyburide, Glipizide, and Glimepiride are examples of sulfonylureas.
Glinides are a newer class of oral hypoglycemic drugs that act by stimulating insulin secretion from the beta cells of the pancreas in response to glucose[4]. They have a rapid onset of action and a shorter duration of action compared to sulfonylureas, which makes them useful for controlling postprandial glucose excursions. Examples of glinides include Repaglinide and Nateglinide.
Research has demonstrated that both sulfonylureas and glinides are effective in decreasing blood glucose levels and enhancing glycemic control in individuals with type 2 diabetes. Nevertheless, they also carry a potential risk of hypoglycemia, which can be a severe adverse effect leading to seizures, coma, or even fatality. Furthermore, both drug classifications have been associated with weight gain and an increased possibility of cardiovascular events, although the findings are not consistent.
The selection of oral hypoglycemic medication depends on various factors, including the patient’s clinical characteristics, preferences, and the potential benefits and risks of each drug. Sulfonylureas and glinides are both effective options for regulating blood glucose levels in type 2 diabetes patients, but the decision of medication should be based on an individual basis.
Mechanisms of Action
Sulfonylureas exert their effect by attaching to specific receptors called ATP-sensitive potassium channels (KATP channels) located on the beta cells of the pancreas[5]. This binding triggers the depolarization of the cell membrane, followed by the secretion of insulin. Furthermore, sulfonylureas increase insulin sensitivity in peripheral tissues, improving glucose uptake in muscles and adipose tissue. The first and second generations of sulfonylureas differ in terms of duration and hypoglycemia risk, with the latter having a longer action duration and lower hypoglycemia risk.
On the other hand, glinides work by binding to another type of receptor called sulfonylurea receptors (SURs) present on beta cells of the pancreas, resulting in cell membrane depolarization and insulin secretion. However, glinides have a faster onset of action and shorter duration of effect than sulfonylureas, which makes them effective in controlling postprandial glucose surges. Glinides have a weaker impact on peripheral insulin sensitivity than sulfonylureas.
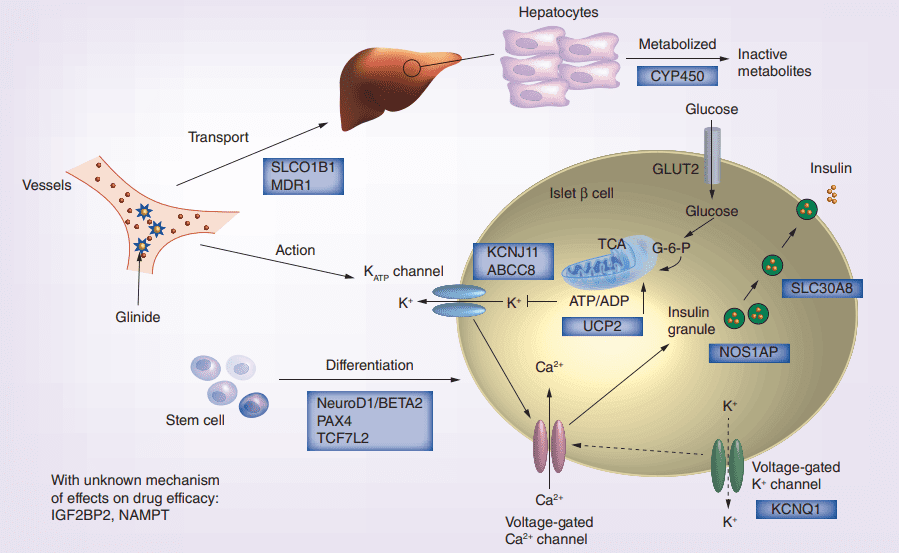
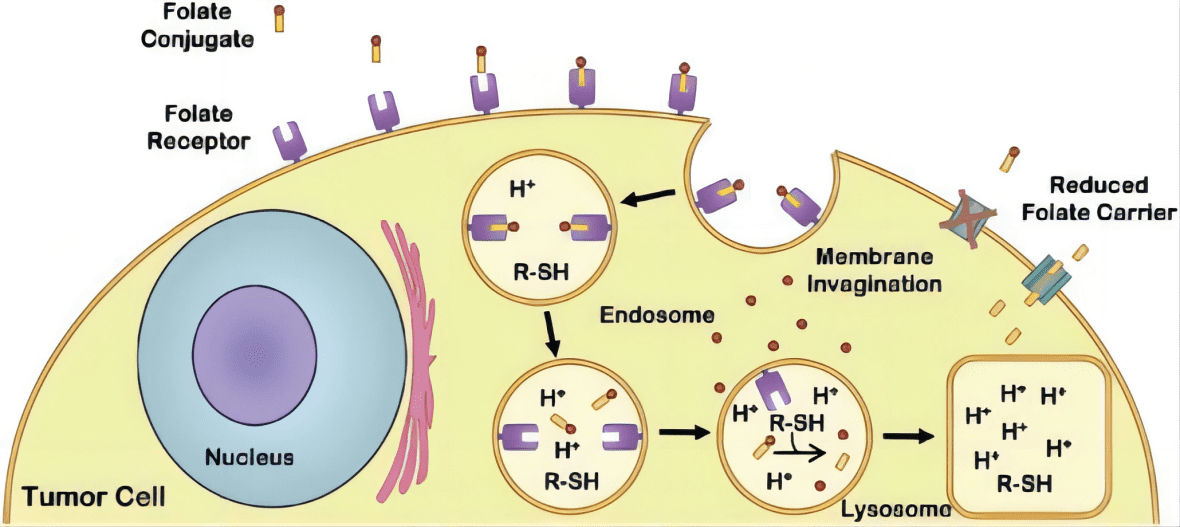
Figure 2 Glinides mechanism of action and the potential effect of pharmacogenomics gene on drug efficacy.[4]
The pharmacokinetics of sulfonylureas and glinides differ significantly. Sulfonylureas are metabolized in the liver and excreted through the urine, while glinides undergo liver metabolism and are eliminated via fecal excretion. Glinides have a shorter half-life compared to sulfonylureas, which can be beneficial for patients who need to adjust their dosage based on their meal schedules.
Another point of contrast between sulfonylureas and glinides is their potential for drug interactions. Sulfonylureas can interact with various medications, such as certain antibiotics and nonsteroidal anti-inflammatory drugs (NSAIDs), which can raise the risk of hypoglycemia. In comparison, glinides have a lower likelihood of drug interactions relative to sulfonylureas.
Both sulfonylureas and glinides are efficacious in reducing blood glucose levels and improving glycemic control in patients with type 2 diabetes. Nonetheless, sulfonylureas have been linked to a greater risk of hypoglycemia, particularly in older patients or those with renal impairment, while glinides may be more effective than sulfonylureas at controlling postprandial glucose excursions, according to some studies. However, the evidence on this matter is not conclusive.
In general, both sulfonylureas and glinides are effective choices for managing blood glucose levels in patients with type 2 diabetes, and the decision to use either drug depends on various factors, such as the patient’s clinical characteristics, preferences, and the potential benefits and risks of each medication.
Pharmacokinetics
Pharmacokinetics refers to the study of the absorption, distribution, metabolism, and elimination of drugs in the body. Understanding the pharmacokinetics of sulfonylureas and glinides is important for optimizing their efficacy and minimizing their potential side effects.
Sulfonylureas are well-absorbed after oral administration, with peak plasma concentrations typically reached within 1-2 hours. The bioavailability of sulfonylureas varies depending on the specific drug and formulation, with some drugs having higher bioavailability than others. For example, glimepiride has a bioavailability of approximately 100%, while glyburide has a bioavailability of only 50-80%. Sulfonylureas are highly protein-bound in the plasma, which can affect their distribution to tissues[6].
Sulfonylureas are extensively metabolized in the liver, primarily by the cytochrome P450 (CYP) enzyme system. The metabolites of sulfonylureas are mainly excreted in the urine, although some drugs are also excreted in the feces. The elimination half-life of sulfonylureas varies depending on the specific drug, with some drugs having longer half-lives than others. For example, chlorpropamide has a half-life of approximately 40-60 hours, while glipizide has a half-life of approximately 2-4 hours.
In contrast, glinides are rapidly absorbed after oral administration, with peak plasma concentrations typically reached within 0.5-1 hour. The bioavailability of glinides is generally high, with nateglinide and repaglinide having bioavailabilities of approximately 73% and 56%, respectively. Glinides are also highly protein-bound in the plasma, which can affect their distribution to tissues.
Glinides are metabolized in the liver, primarily by the CYP enzyme system. The metabolites of glinides are mainly excreted in the feces, although some drugs are also excreted in the urine. The elimination half-life of glinides is generally shorter than that of sulfonylureas, with nateglinide and repaglinide having half-lives of approximately 1-2 hours and 1 hour, respectively.
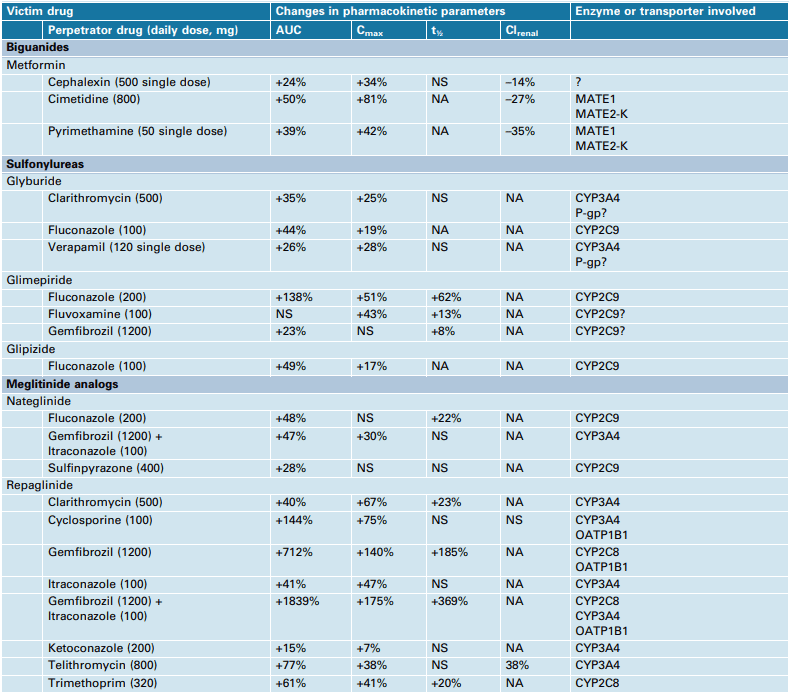
Figure 3 Effects of CYP enzyme and/or transporter inhibiting drugs on the pharmacokinetics of oral antidiabetic drugs in healthy subjects[7]
Factors such as age, renal function, and drug interactions can affect the pharmacokinetics of sulfonylureas and glinides. In patients with renal impairment, the clearance of sulfonylureas and glinides may be reduced, which can increase the risk of hypoglycemia. In addition, certain drugs, such as antifungal agents and macrolide antibiotics, can inhibit the metabolism of sulfonylureas and glinides, which can also increase the risk of hypoglycemia.
Adverse Effects
Adverse effects are a significant consideration when using sulfonylureas and glinides in the management of type 2 diabetes. These drugs can have a range of side effects, some of which can be serious.
Hypoglycemia is a common adverse effect of sulfonylureas and glinides. These drugs stimulate insulin secretion from the pancreas, which can cause blood glucose levels to drop too low[8]. Symptoms of hypoglycemia can include confusion, dizziness, sweating, and weakness. Severe hypoglycemia can lead to seizures, coma, and even death.
Weight gain is another common adverse effect of sulfonylureas and glinides. This is thought to be due in part to the drugs’ effects on insulin secretion, which can lead to increased appetite and food intake.
Sulfonylureas have been associated with an uncertain risk of cardiovascular events, with conflicting evidence from different studies. Some studies suggest an increased risk of heart attack, stroke, and cardiovascular death, while others do not show a significant increase. Glinides may also have some cardiovascular effects, although the evidence is less definitive than for sulfonylureas[9].
Both classes of drugs can also cause other adverse effects, such as gastrointestinal symptoms like nausea, vomiting, and diarrhea, as well as skin rash and allergic reactions. In rare cases, liver toxicity can occur, leading to jaundice, liver failure, and other severe complications.
Clinical Use
Sulfonylureas and glinides are both classes of oral hypoglycemic agents used to manage type 2 diabetes, although there are notable differences between them. Sulfonylureas are typically prescribed as a second-line therapy after metformin and function by stimulating insulin release from beta cells in the pancreas. There are first-generation sulfonylureas such as chlorpropamide and tolazamide, and second-generation sulfonylureas such as glyburide, glipizide, and glimepiride, with varying duration of action and potency[10]. Although generally well-tolerated, sulfonylureas carry a risk of hypoglycemia, weight gain, and other adverse effects.
Glinides are another class of oral hypoglycemic agents that work by stimulating insulin secretion from the pancreatic beta cells. Like sulfonylureas, glinides are typically used as second-line therapy after metformin in the management of type 2 diabetes. They are available in two formulations: Repaglinide and Nateglinide. These drugs have a more rapid onset and shorter duration of action than sulfonylureas, making them particularly useful for controlling postprandial glucose excursions. Glinides are generally well-tolerated but can cause hypoglycemia and weight gain, and may have some effects on cardiovascular function.
In terms of efficacy, sulfonylureas, and glinides are generally comparable. Both drug classes can help to lower HbA1c levels by about 1-2%, although sulfonylureas may be slightly more effective in some patients. However, sulfonylureas have a longer duration of action and may be associated with a higher risk of hypoglycemia than glinides. Glinides, on the other hand, have a more rapid onset and shorter duration of action, which may make them better suited for patients with irregular meal schedules or who are at risk of postprandial hyperglycemia.
Potential Role in the Management of Type 2 Diabetes
Sulfonylureas and glinides have been established as effective and safe treatments for type 2 diabetes over several decades of clinical use. However, ongoing research is exploring potential future directions for these drugs in diabetes management. One area of interest is combining them with other medications, such as metformin, GLP-1 agonists, or SGLT-2 inhibitors, to enhance glycemic control and reduce hypoglycemia risk. This strategy may also offer additional benefits, such as weight loss or cardiovascular protection, and could shape the future use of these drugs in diabetes management[11].
Figure 4 Type 2 diabetes[12]
Researchers are investigating the potential of using sulfonylureas and glinides in specific patient groups. Studies have shown that sulfonylureas could be more effective in older patients or those who have had diabetes for a shorter duration, while glinides may be better suited for patients with postprandial hyperglycemia or those with irregular meal schedules.
Furthermore, scientists are exploring the cardioprotective properties of these medications. Although sulfonylureas and glinides have historically been linked with some cardiovascular risks, recent studies suggest they may also have positive effects on cardiovascular function by improving endothelial function or reducing inflammation. Nevertheless, additional research is necessary to understand the full extent of these benefits and how they can be used to manage type 2 diabetes.
Overall, sulfonylureas and glinides continue to be essential options for managing type 2 diabetes. Despite some potential drawbacks, such as hypoglycemia and weight gain, these drugs can still enhance glycemic control and reduce the risk of complications associated with uncontrolled diabetes. Ongoing research into their use in combination with other medications or in specific patient populations may help refine their role in managing type 2 diabetes.
Summary
The article discusses the mechanisms of action, pharmacokinetics, adverse effects, clinical use, and potential role in managing type 2 diabetes for both sulfonylureas and glinides. Both drugs are effective in lowering blood glucose levels and can aid in improving glycemic control in type 2 diabetes patients. Nonetheless, they have distinct mechanisms of action, pharmacokinetics, and adverse effects. Sulfonylureas have a longer half-life and are associated with a higher risk of hypoglycemia and weight gain, while glinides have a shorter half-life and are associated with a lower risk of these adverse effects. The efficacy and safety of both drugs have been well-established through numerous clinical studies over several decades. Current research is exploring their potential use in combination with other medications or in specific patient populations. Overall, both drugs remain important options for managing type 2 diabetes, and ongoing research may help to refine their role in this context.
Reference
[1] American Diabetes Association. (2013). Position Statement: Standard of Medical Care in Diabetes-2013. American Diabetes Associations, 68-73.