Palbociclib - A Breakthrough CDK4/6 Inhibitor for Breast Cancer Treatment
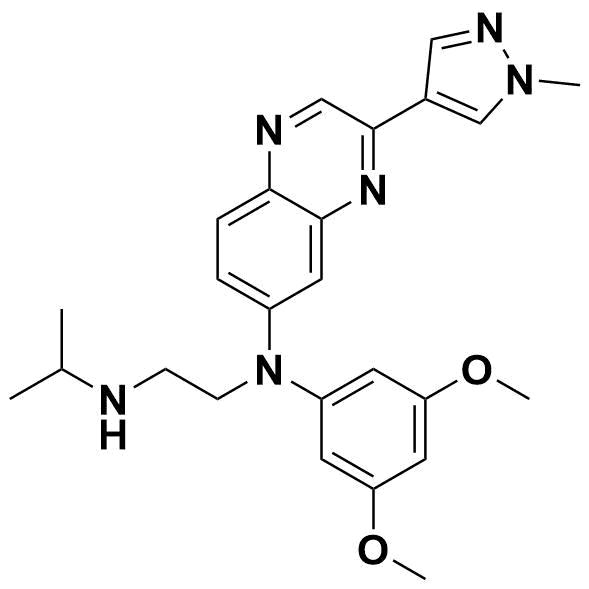
Palbociclib (trade name: Ibrance) is a medication used to treat breast cancer. It is a type of drug called a cyclin-dependent kinase (CDK) inhibitor, which works by blocking certain proteins that cancer cells need to divide and grow. Palbociclib is typically used in combination with other medications to treat hormone receptor-positive, HER2-negative advanced or metastatic breast cancer in postmenopausal women or men. It is usually taken orally in the form of capsules and was approved by the U.S. Food and Drug Administration (FDA) in 2015[1].
What is breast cancer?
Breast cancer is a major global health concern and the leading cause of cancer-related deaths among women worldwide. Annually, around 2.3 million new cases of breast cancer are diagnosed, resulting in about 685,000 deaths. Despite improvements in early detection and treatment, there is still a significant need for new and effective therapies, especially for patients with advanced or metastatic disease[2].
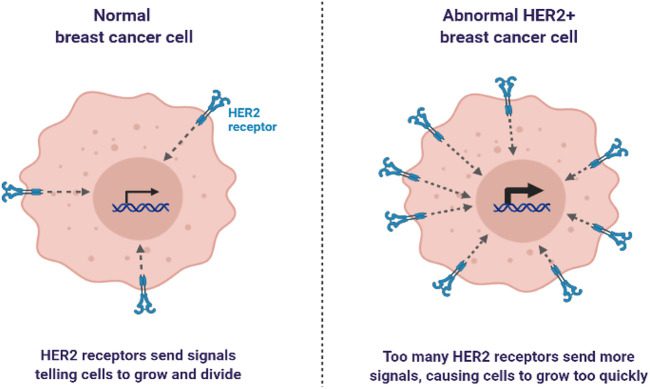
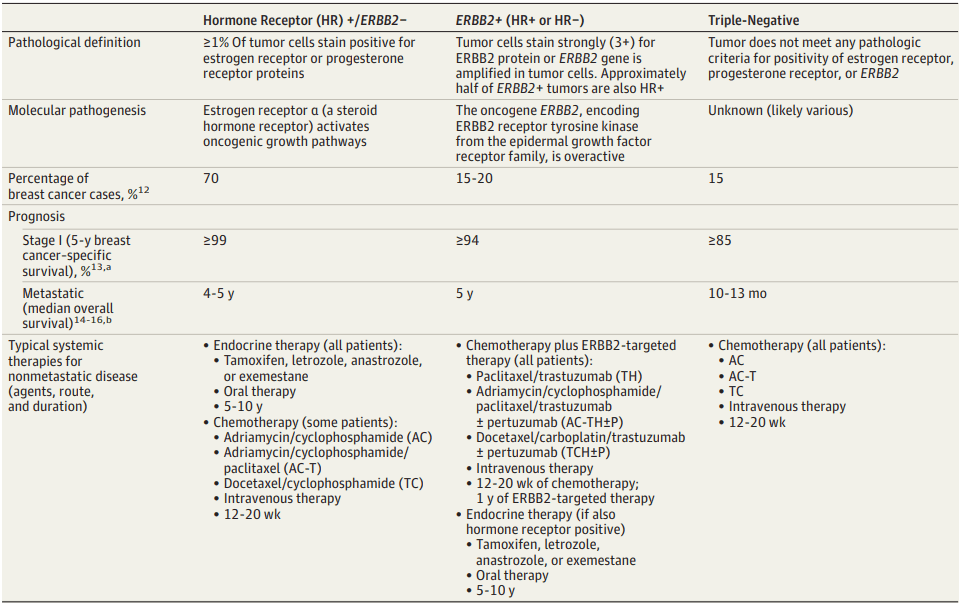
Breast cancer develops in the breast cells and can affect both men and women, although it’s more prevalent in women. The cancer can be classified based on the presence or absence of hormone receptors (HRs) and human epidermal growth factor receptor 2 (HER2) expression. The most common type is hormone receptor-positive (HR+), accounting for around 70% of cases. HER2-positive breast cancer represents about 15-20% of cases, while triple-negative breast cancer (TNBC) accounts for approximately 10-15%. TNBC is a particularly aggressive and challenging form of the disease because it lacks the hormone receptors and HER2 expression targeted by many current therapies[3].
Although breast cancer treatment has advanced, there is still a pressing need for new and effective therapies, especially for patients with advanced or metastatic disease. The available treatments can cause significant side effects, and many patients eventually develop resistance to therapy. Furthermore, treatment options for patients with TNBC are limited, which is associated with poor prognosis and high recurrence rates. Developing new therapies for breast cancer is complex and challenging, but recent advancements in our understanding of breast cancer biology have led to the identification of new therapy targets. For instance, CDK4/6 inhibitors, such as palbociclib, have shown significant clinical activity when combined with hormonal therapy, and are now approved for treating advanced or metastatic HR+ breast cancer. Moreover, there is a growing interest in using immunotherapy for treating breast cancer, especially for TNBC patients. Pembrolizumab and atezolizumab, immune checkpoint inhibitors, have shown promising results in early-phase clinical trials and are currently undergoing larger clinical trials.
Table 1. Prevalence, Prognosis, and Therapeutic Options for the 3 Breast Cancer Subtypes[3]
To conclude, breast cancer remains a significant global health concern, emphasizing the necessity for new and effective therapies. Thanks to recent progress in comprehending breast cancer’s biology, we have discovered new therapy targets, and immunotherapy development for treating breast cancer is gaining traction. However, continuing research and collaboration between clinicians, researchers, and industry partners are necessary for developing new therapies for breast cancer.
How does Palbociclib work in human body?
Palbociclib is a selective inhibitor of cyclin-dependent kinases 4 and 6 (CDK4/6), which are critical regulators of the cell cycle. In breast cancer cells, the CDK4/6 pathway is frequently dysregulated, leading to uncontrolled cell proliferation and tumor growth. Palbociclib works by binding to and inhibiting the activity of CDK4/6, thereby preventing the progression of cells through the cell cycle and inducing cell cycle arrest.
Mechanism of action
The CDK4/6 pathway regulates the cell cycle. Cyclin D1 activates CDK4/6, which phosphorylates RB. Phosphorylated RB releases E2F transcription factors that promote cell cycle progression. Dysregulated CDK4/6 in breast cancer cells leads to uncontrolled cell proliferation. Palbociclib inhibits CDK4/6 by binding to its ATP-binding site, preventing phosphorylation of RB and blocking cell cycle progression[4]. This results in cell cycle arrest, decreased cell proliferation, and inhibition of tumor growth. Palbociclib is most effective in HR+ breast cancer and can be combined with hormonal therapy. Preclinical studies suggest it may enhance the activity of chemotherapy, radiation therapy, and immunotherapy against tumors.[5]
Pharmacokinetics
Palbociclib is administered orally, with a recommended dose of 125 mg once daily for 21 days followed by 7 days off treatment. Palbociclib is metabolized primarily by the cytochrome P450 3A4 (CYP3A4) enzyme, and co-administration of strong CYP3A4 inhibitors or inducers should be avoided. Palbociclib exhibits dose-proportional pharmacokinetics, with a median time to peak plasma concentration of approximately 6 hours. The elimination half-life of Palbociclib is approximately 30 hours, and steady-state concentrations are typically achieved after 8 weeks of treatment.[6,7]
Pharmacodynamics
Palbociclib, an effective treatment for HR+ breast cancer, has shown promising results in inducing cell cycle arrest in breast cancer cell lines and xenograft models. Clinical studies have confirmed its activity in patients with advanced HR+ breast cancer, where it significantly prolongs progression-free survival when used in combination with letrozole. The drug has also shown efficacy in treating other tumor types, such as liposarcoma and mantle cell lymphoma, leading to its approval for liposarcoma treatment in 2019. Palbociclib is well tolerated, with the most common adverse events being fatigue, nausea, and neutropenia. Although neutropenia is the most common grade 3 or 4 adverse event, patients should be regularly monitored for signs of infection. Furthermore, Palbociclib has the potential to cause QT prolongation and should be used with caution in patients with a history of QT prolongation or those taking medications that can prolong the QT interval.[8]
Palbociclib is a promising treatment for HR+ breast cancer, as it selectively inhibits the CDK4/6 pathway, induces cell cycle arrest, and prevents cell cycle progression. Extensive studies have characterized its pharmacokinetic and pharmacodynamic profiles in both preclinical and clinical settings, demonstrating its efficacy and generally well-tolerated nature. The most common adverse event associated with Palbociclib is neutropenia. Overall, the development of Palbociclib is a significant advance in breast cancer treatment, offering a new and promising option for patients with this disease.
Clinical trials of Palbociclib
Phase I studies
The safety profile and maximum tolerated dose (MTD) of Palbociclib were evaluated in a phase I study involving patients with advanced solid tumors in 2011. The study determined that the MTD of Palbociclib was 200 mg/day and identified neutropenia, fatigue, and nausea as the most commonly reported adverse events. Furthermore, the study showed initial signs of clinical activity, with 3 patients experiencing partial responses and 10 patients achieving stable disease.[6,7]
Phase II studies
Following promising results from the phase I study, several phase II studies were conducted to further evaluate the safety and efficacy of Palbociclib in combination with hormonal therapy in patients with HR+ breast cancer. In 2013, the first phase II study evaluated Palbociclib in combination with letrozole in postmenopausal women with advanced breast cancer. The study demonstrated a significant improvement in progression-free survival (PFS) for patients receiving Palbociclib plus letrozole compared to letrozole alone.[9]
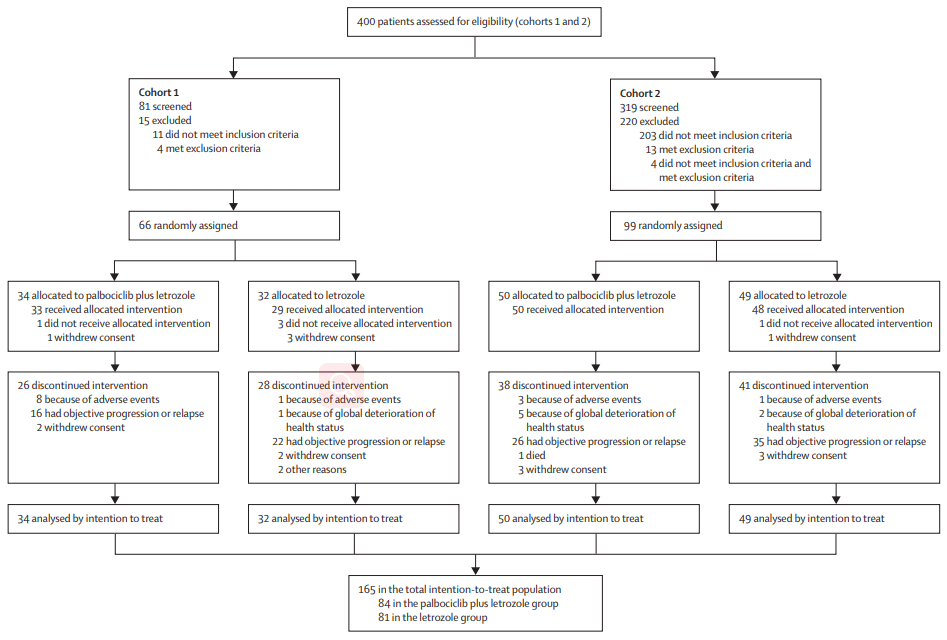
Figure 1 Trial profile of phase II study[9]
Subsequent phase II studies evaluated Palbociclib in combination with other hormonal therapies, such as fulvestrant and tamoxifen. These studies also showed a significant improvement in PFS and overall response rate compared to hormonal therapy alone(Figure 1). Overall, the clinical activity of Palbociclib in HR+ breast cancer was confirmed, leading to its approval for this indication in 2015.
Phase III studies
Palbociclib in combination with letrozole was evaluated in the first phase III study, PALOMA-2, a randomized, double-blind, placebo-controlled trial conducted in postmenopausal women with advanced breast cancer. The study demonstrated a significant improvement in progression-free survival (PFS) in patients receiving Palbociclib plus letrozole compared to letrozole alone, with a median PFS of 24.8 months in the Palbociclib arm versus 14.5 months in the placebo arm. The results of PALOMA-2 led to the approval of Palbociclib in combination with letrozole as a first-line treatment for HR+ breast cancer.
In the second phase III study, PALOMA-3, Palbociclib was evaluated in combination with fulvestrant in patients with HR+ breast cancer who had progressed on prior hormonal therapy. The study demonstrated a significant improvement in PFS in patients receiving Palbociclib plus fulvestrant compared to fulvestrant alone, with a median PFS of 9.5 months in the Palbociclib arm compared to 4.6 months in the placebo arm. The results of PALOMA-3 supported the approval of Palbociclib in combination with fulvestrant as a second-line treatment for HR+ breast cancer.
The MONALEESA-2 phase III study focused on evaluating the efficacy of Palbociclib in combination with letrozole in postmenopausal women with advanced breast cancer. The study demonstrated a significant improvement in progression-free survival (PFS) in patients receiving Palbociclib plus letrozole compared to letrozole alone, with a median PFS of 25.3 months in the Palbociclib arm compared to 16.0 months in the placebo arm. The results of MONALEESA-2 further reinforce the clinical activity of Palbociclib in combination with hormonal therapy for patients with HR+ breast cancer.[10-12]
Collectively, the phase III studies have solidified the position of Palbociclib as a standard of care for HR+ breast cancer patients. The approval of Palbociclib in combination with letrozole as a first-line treatment, and fulvestrant as a second-line treatment, reflects the significant improvement in PFS achieved by adding Palbociclib to the standard of care for HR+ breast cancer patients. Ongoing studies are investigating the efficacy of Palbociclib in combination with other therapies, and it will be interesting to see how these findings contribute to the evolving standard of care for HR+ breast cancer. Nonetheless, the impact of Palbociclib in improving outcomes for patients with HR+ breast cancer cannot be overstated.
Is Palbociclib safety?
The most common adverse events associated with Palbociclib are neutropenia, fatigue, nausea, infections, and alopecia. Neutropenia, a reduction in white blood cells, is the most common adverse event and can lead to serious infections. The incidence of grade 3/4 neutropenia is approximately 66%, but febrile neutropenia is rare. Other adverse events, such as fatigue and nausea, are typically mild to moderate in severity.[13]
Palbociclib is primarily metabolized by the liver through the cytochrome P450 (CYP) pathway, particularly CYP3A4. Therefore, drugs that induce or inhibit CYP3A4 may affect the pharmacokinetics of Palbociclib. Strong CYP3A4 inhibitors, such as ketoconazole and ritonavir, may increase the exposure of Palbociclib, while strong CYP3A4 inducers, such as rifampin and phenytoin, may decrease the exposure of Palbociclib. It is recommended to avoid strong CYP3A4 inhibitors or inducers when administering Palbociclib.
Palbociclib is contraindicated in patients with severe hepatic impairment and in combination with strong CYP3A4 inhibitors that may increase the exposure of Palbociclib. It is also contraindicated in patients who are allergic to any of the components of the drug. Palbociclib is not recommended in patients with a history of interstitial lung disease or pneumonitis. It is also not recommended during pregnancy or breastfeeding due to the potential risk to the fetus or infant.
Palbociclib is contraindicated in patients with severe hepatic impairment and in combination with strong CYP3A4 inhibitors that may increase the exposure of Palbociclib. It is also contraindicated in patients who are allergic to any of the components of the drug. Palbociclib is not recommended in patients with a history of interstitial lung disease or pneumonitis. It is also not recommended during pregnancy or breastfeeding due to the potential risk to the fetus or infant.[14]
Palbociclib is a medication that is used to treat certain types of cancer. However, there are several precautions and contraindications that need to be considered before prescribing it to patients. Patients with severe hepatic impairment and those taking strong CYP3A4 inhibitors are contraindicated from taking Palbociclib as it may increase the drug’s exposure, leading to potential adverse effects. Additionally, patients who have a known allergy to any of the drug’s components should avoid taking Palbociclib. Furthermore, patients with a history of interstitial lung disease or pneumonitis should not be prescribed Palbociclib. Lastly, pregnant or breastfeeding women should not use this medication due to potential risks to the fetus or infant. It is important to thoroughly evaluate a patient’s medical history and current medication regimen before prescribing Palbociclib to ensure their safety and avoid any potential adverse effects.
Current research of Palbociclib
Wedam et al. provides an overview of the FDA approval of Palbociclib for the treatment of metastatic breast cancer in male patients. This paper discuss the background and epidemiology of male breast cancer, which is a rare but important disease. Although male breast cancer comprises only 1% of all breast cancer cases, it is associated with a higher mortality rate than female breast cancer.[15]
It’s important to develope effective treatment options for male breast cancer patients, particularly those with advanced disease. Palbociclib, a cyclin-dependent kinase 4/6 inhibitor, has demonstrated significant clinical activity in female patients with hormone receptor-positive (HR+) and HER2-negative metastatic breast cancer. Therefore, the efficacy and safety of Palbociclib were evaluated in male patients with HR+ and HER2-negative metastatic breast cancer in the phase III PALOMA-3 trial. The trial enrolled 521 male patients with metastatic breast cancer who had previously received endocrine therapy. The patients were randomized to receive either Palbociclib in combination with fulvestrant or placebo in combination with fulvestrant. The primary endpoint of the trial was progression-free survival (PFS). The results of the PALOMA-3 trial showed that Palbociclib in combination with fulvestrant significantly improved PFS compared to placebo in combination with fulvestrant. The median PFS was 11.5 months in the Palbociclib group and 4.6 months in the placebo group. The improvement in PFS was observed regardless of age, race, or the presence of liver or lung metastases. Also, the safety profile of Palbociclib in male patients was consistent with that observed in female patients. (Figure 2)
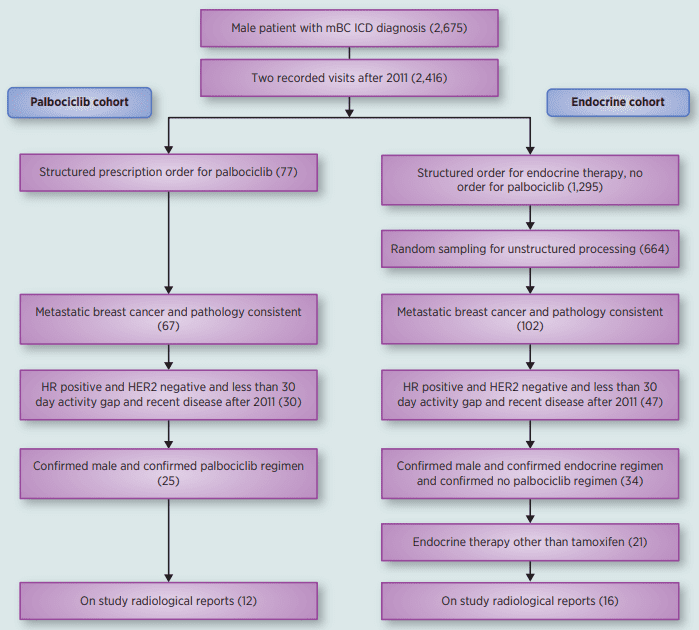
Figure 2 Flatiron Database Analysis Consort Diagram.
The FDA’s approval of Palbociclib marks a significant advancement in treating the rare disease of male breast cancer with HR+ and HER2-negative metastases. Combining Palbociclib with fulvestrant offers a fresh treatment alternative for males who have already undergone endocrine therapy. However, additional research is required to establish the ideal sequencing of Palbociclib and other therapies for male breast cancer.
Reference:
[1] Dhillon, S. (2015). Palbociclib: first global approval. Drugs, 75, 543-551.
[3] Waks, A. G., & Winer, E. P. (2019). Breast cancer treatment: a review. Jama, 321(3), 288-300.