Dopamine Receptor and Cancer
Dopamine receptors are a type of G protein-coupled receptor that binds dopamine, a neurotransmitter in the brain. There are five different subtypes of dopamine receptors, labeled as D1 through D5. They are widely distributed throughout the brain and play important roles in various physiological and behavioral processes, including movement, motivation, reward, addiction, and mood regulation. Dysfunction in dopamine receptors has been implicated in several neurological and psychiatric disorders, such as Parkinson’s disease, schizophrenia, addiction, etc. Recently, several pieces of the literature showed it may be a potential cancer treatment target.
How does Dopamine receptor work in human body?
Dopamine receptors are an essential part of the human body’s neurochemistry, playing a crucial role in the regulation of a variety of physiological functions. Dopamine receptors are G-protein coupled receptors that bind the neurotransmitter dopamine, which is a chemical messenger that is produced in the brain and is involved in various physiological and behavioral processes. There are five types of dopamine receptors, each of which plays a distinct role in different areas of the brain and body.
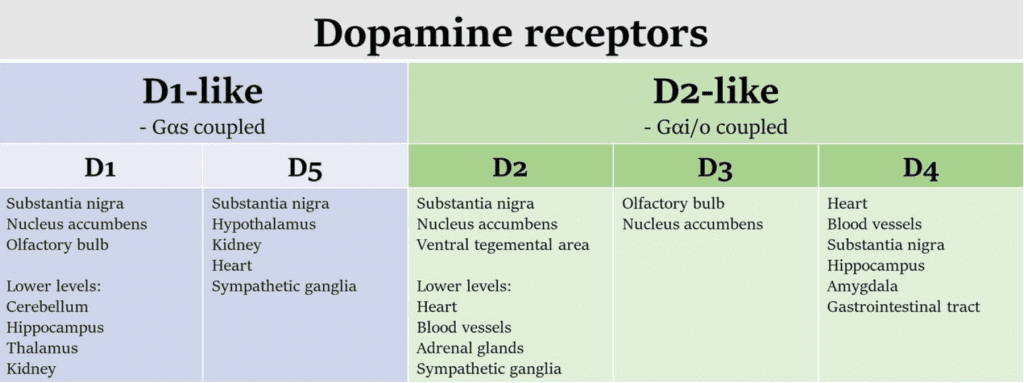
One of the primary functions of dopamine receptors is to regulate movement and motor control[1]. The dopamine D1 receptor is involved in the activation of the motor cortex, while the dopamine D2 receptor plays a role in the regulation of the motor output. Dysfunctions in dopamine receptors can lead to movement disorders such as Parkinson’s disease, where the death of dopamine-producing cells in the brain leads to a deficiency in dopamine and impaired motor function(Table 1).
Table 1 Dopamine receptors are G protein-coupled receptors, which are divided into the D1- and D2-like families. Some tissues of interest where these receptors are expressed are included here.[2]
Dopamine receptors are also involved in the regulation of reward and motivation. The dopamine D1 receptor is thought to be involved in the reward pathway, which is activated when we experience pleasure, while the dopamine D2 receptor is involved in motivation and goal-directed behavior. Dysfunction in dopamine receptors has been implicated in addiction and substance abuse, as the dopamine reward pathway is activated by drugs of abuse, leading to cravings and compulsive drug-seeking behaviors [3].
Dopamine receptors also play a role in cognitive functions such as learning and memory. The dopamine D1 receptor is involved in the formation of long-term memory, and the dopamine D2 receptor is thought to play a role in working memory and attentional control[4]. Dysfunction in dopamine receptors has been implicated in various neuropsychiatric disorders such as schizophrenia, where there is an imbalance in dopamine activity in the brain leading to cognitive deficits[5].
In addition to its role in the brain, dopamine also plays a role in the regulation of cardiovascular function. The dopamine D1 receptor is expressed in the renal vasculature and is involved in the regulation of blood pressure, while the dopamine D2 receptor is expressed in the heart and is involved in the regulation of heart rate [6]. Dysfunction in dopamine receptors has been implicated in cardiovascular disorders such as hypertension and heart failure.
Apart from these functions, dopamine receptors have also been found to play a role in other physiological functions, such as thermoregulation, immune response, endocrine regulation, and pain processing [7]. However, the precise mechanisms and roles of dopamine receptors in these processes are still being studied.
Overall, dopamine receptors play a critical role in the regulation of a variety of physiological functions in the human body, including movement, reward and motivation, cognition, and cardiovascular function. Dysfunctions in dopamine receptors can lead to a variety of disorders and diseases, including movement disorders, addiction, neuropsychiatric disorders, and cardiovascular disorders.
If Dopamine Receptor can be used for cancer treatment?
While dopamine receptors are primarily known for their role in the central nervous system, there is some evidence to suggest that they may also play a role in cancer. Specifically, certain dopamine receptors, such as the dopamine D2 receptor, have been found to be expressed in various types of cancer cells, including breast, prostate, lung, and gastrointestinal cancers. As a result, there has been interest in exploring whether dopamine receptor agonists or antagonists, which can modulate dopamine receptor activity, could be used as a potential cancer treatment[8-11].
Dopamine D2 receptors have been identified as potential targets for cancer therapy due to their involvement in cancer cell growth, proliferation, and survival. Dopamine D2 receptor-targeting agents have shown promising anticancer effects in preclinical and clinical studies, making them an attractive avenue for the development of novel cancer therapeutics.
Haloperidol, an antipsychotic drug, is one of the most widely investigated dopamine D2 receptor-targeting agents. Studies have confirmed its ability to suppress the proliferation of numerous cancer cell lines, such as breast, prostate, lung, and colon cancers, both in vitro and in vivo (Figure 1). The anticancer activity of haloperidol is thought to be caused by the blockade of dopamine D2 receptors on cancer cells, leading to the inhibition of downstream signaling pathways involved in cell growth and survival. Another category of dopamine D2 receptor-targeting agents that have been studied for their potential anticancer properties are dopamine agonists[12]. These drugs activate dopamine receptors and have been shown to impede cancer cell growth and induce apoptosis in a variety of cancers, including breast, prostate, and neuroblastoma. For example, bromocriptine, a dopamine agonist, has been shown to curb the growth of triple-negative breast cancer cells by decreasing cyclin-D1 expression.
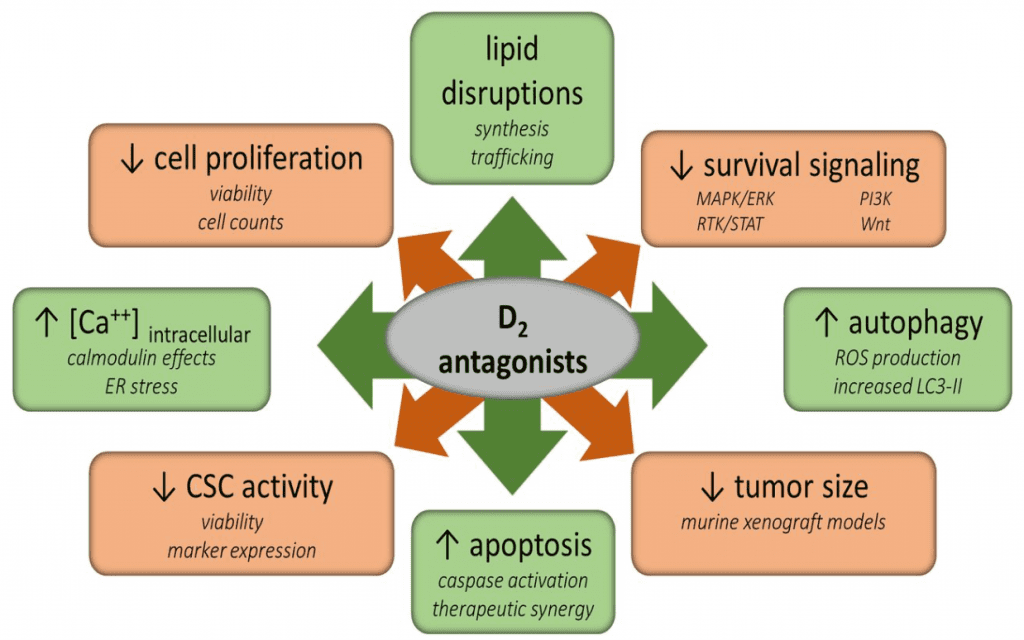
Figure 1 Treatment with D2 antagonists affects many vital metabolic processes within cancer cells and tumors. Cancer stem cell-like activities, survival signaling, and proliferation are reduced by treatment. However, intracellular calcium levels, autophagy, and apoptosis are increased. Additionally, lipid synthesis and trafficking are disrupted. The direct mechanisms by which these alterations occur is not currently known, but these compounds may ultimately lead to cell death through these or other pathways.[2]
Despite the promising results of preclinical and clinical studies, there are still challenges and limitations in the development of dopamine D2 receptor-targeting agents as cancer therapeutics. One major challenge is the potential for these drugs to cause side effects due to their effects on the central nervous system, as dopamine receptors are widely distributed throughout the brain. Additionally, individual differences in dopamine receptor expression and signaling may impact the efficacy of these drugs in different cancer types and in different patient populations. Overall, dopamine D2 receptor-targeting agents represent a promising avenue for the development of novel cancer therapeutics. Further research is needed to fully understand the mechanisms of action of these drugs and to optimize their clinical efficacy and safety.
Researchs about Dopamine Receptor used for cancer treatment?
Dopamine D2 receptors have been confirmed of extensive research in various types of cancer, such as breast, lung, prostate, colorectal, and pancreatic cancer[13-16]. Dysregulation of the dopamine D2 receptor has been linked to the development and progression of cancer, and its expression and function in this context have been thoroughly investigated.
One of the primary mechanisms by which dopamine D2 receptors contribute to cancer development and progression is by activating downstream signaling pathways that regulate cell growth and survival. When dopamine activates the dopamine D2 receptor, it can activate the Gαi/o protein complex, which inhibits adenylyl cyclase and lowers intracellular cAMP levels. This, in turn, can activate various downstream effectors, such as the MAPK/ERK pathway, the PI3K/Akt pathway, and the mTOR pathway[17], all of which promote cancer cell growth and survival.
In addition to their direct effects on cancer cell growth and survival, dopamine D2 receptors have also been shown to regulate angiogenesis and the tumor microenvironment. The dopamine D2 receptor has been found to be expressed on endothelial cells and to regulate the production of angiogenic factors, such as VEGF, through the activation of downstream signaling pathways. This can lead to the promotion of angiogenesis and the development of a tumor-supportive microenvironment.
Furthermore, dopamine D2 receptors have been found to be involved in the regulation of immune function in the tumor microenvironment. The dopamine D2 receptor has been shown to be expressed on immune cells, including T cells and macrophages, and to regulate immune cell activation and cytokine production. This can lead to the suppression of anti-tumor immune responses and the promotion of a tumor-supportive microenvironment.
Although the expression and function of dopamine D2 receptors in various types of cancer have been extensively studied, there are still many unanswered questions regarding their role in cancer development and progression. It remains unclear how the expression and signaling of dopamine D2 receptors are regulated in cancer cells, and how this regulation may differ among different cancer types and stages. Furthermore, the effects of dopamine D2 receptor-targeting agents on the tumor microenvironment and immune function are not fully understood and require further investigation.
Nonetheless, studies have shown that dopamine D2 receptors play a significant role in cancer development and progression. The dysregulation of these receptors can activate downstream signaling pathways involved in cell growth and survival, as well as regulate angiogenesis and the tumor microenvironment. To fully understand the mechanisms by which dopamine D2 receptors contribute to cancer development and to develop more effective and targeted therapies for cancer treatment, further research is needed.
Overview of different types of dopamine D2 receptor-targeting agents
Dopamine D2 receptor-targeting agents are a class of drugs that act on the dopamine D2 receptor, either as agonists or antagonists. These agents have been investigated for their potential anticancer effects, based on the role of dopamine D2 receptors in cancer development and progression(Table 2).
Table 2 Ligand affinities of select D2 antagonists, agonists, and functionally selective ligands (nM)[2]
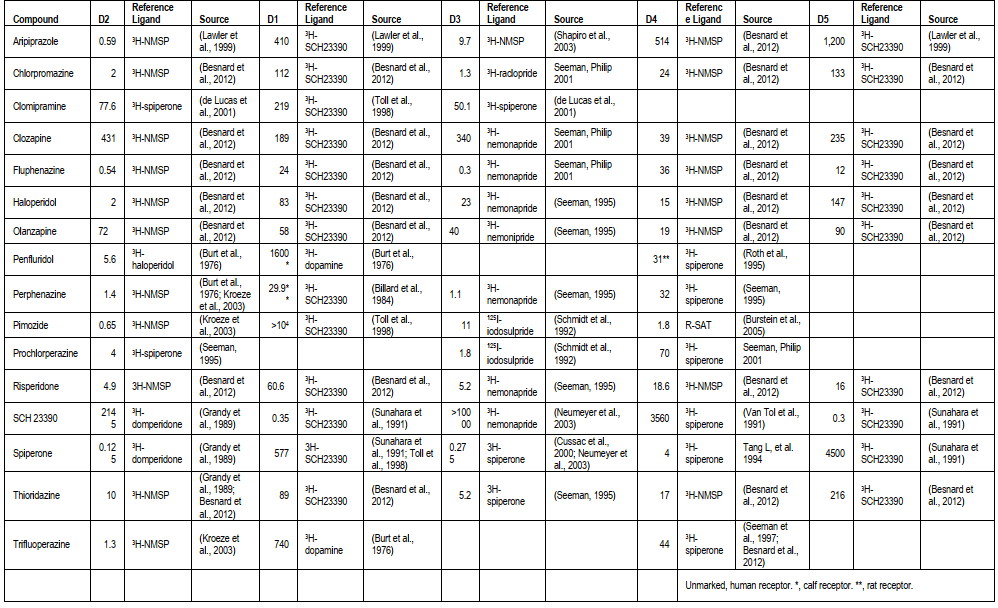
Haloperidol and bromocriptine are two types of dopamine D2 receptor-targeting agents that have been studied for their potential anticancer effects. Haloperidol, a dopamine D2 receptor antagonist commonly used in the treatment of psychiatric disorders, has also been found to inhibit cancer cell growth and induce apoptosis in various types of cancer. On the other hand, dopamine D2 receptor agonists, such as bromocriptine, have also been explored for their anticancer properties. These agents activate dopamine D2 receptors and the downstream signaling pathways involved in cancer cell growth and survival, leading to the inhibition of cancer cell proliferation and the induction of apoptosis in various types of cancer. Preclinical studies have shown that both dopamine D2 receptor antagonists and agonists can reduce tumor growth in animal models of breast, lung, prostate, and pancreatic cancer[18]. These findings suggest that dopamine D2 receptor-targeting agents have potential as a novel therapeutic strategy for the treatment of cancer.
Several preclinical and clinical studies have investigated the anticancer effects of dopamine D2 receptor-targeting agents in various types of cancer. In breast cancer, dopamine D2 receptor antagonists, such as haloperidol, have been shown to inhibit cancer cell proliferation and induce apoptosis in vitro and reduce tumor growth in animal models. Clinical studies have also shown that dopamine D2 receptor antagonists, such as risperidone, can improve the efficacy of chemotherapy in breast cancer patients.
In lung cancer, dopamine D2 receptor antagonists, such as haloperidol and chlorpromazine, have been shown to inhibit cancer cell proliferation and induce apoptosis in vitro and reduce tumor growth in animal models. Clinical studies have also shown that dopamine D2 receptor antagonists, such as metoclopramide, can improve the efficacy of chemotherapy in lung cancer patients.
In prostate cancer, dopamine D2 receptor antagonists, such as haloperidol and risperidone, have been shown to inhibit cancer cell proliferation and induce apoptosis in vitro and reduce tumor growth in animal models. Clinical studies have also shown that dopamine D2 receptor antagonists, such as risperidone, can improve the efficacy of androgen deprivation therapy in prostate cancer patients.
In pancreatic cancer, dopamine D2 receptor agonists, such as bromocriptine, have been shown to inhibit cancer cell proliferation and induce apoptosis in vitro and reduce tumor growth in animal models. Clinical studies have also shown that dopamine D2 receptor agonists, such as cabergoline, can improve the efficacy of chemotherapy in pancreatic cancer patients.
Despite promising preclinical and clinical data, the use of dopamine D2 receptor-targeting agents as anticancer therapies is still in the early stages of development[19]. Further studies are needed to fully understand the mechanisms of action of these agents in different types of cancer and to optimize their use in combination with other therapies. Moreover, the potential side effects of these agents, particularly dopamine D2 receptor antagonists, such as extrapyramidal symptoms, should also be carefully evaluated.
In conclusion, dopamine D2 receptor-targeting agents, including agonists and antagonists, have shown potential as anticancer therapies in various types of cancer. These agents act by targeting the dopamine D2 receptor and the downstream signaling pathways involved in cancer cell growth and survival. However, further studies are needed to optimize their use and to evaluate their potential side effects.
Reference: