What is Gemcitabine
Gemcitabine (CAT: I006946) is a vital chemotherapy drug that has been utilized in cancer therapy for over two decades. Its approval by the FDA in 1996 for the treatment of pancreatic cancer was a significant milestone in cancer treatment, as gemcitabine became an essential part of the treatment arsenal for other cancers like breast, lung, and bladder cancer. Chemotherapy drugs like gemcitabine have played a critical role in improving cancer patient survival rates by shrinking tumors, preventing metastasis, and prolonging lives. With its proven efficacy, gemcitabine has become a valuable tool in the fight against cancer. However, gemcitabine can cause side effects like myelosuppression, nausea, and vomiting, and less common but severe adverse effects like pulmonary toxicity and infusion-related reactions. Careful monitoring and management of these side effects are necessary to maximize the potential benefits of gemcitabine in cancer therapy.
Mechanism of Action:
Gemcitabine functions by disrupting the DNA synthesis and repair process in cancer cells, leading to their destruction. Specifically, gemcitabine is a nucleoside analog, which imitates the structure of a DNA building block. Once inside the cancer cell, gemcitabine is incorporated into the DNA during replication, causing defects in the DNA sequence, which ultimately leads to the cell’s death.(Figure 1)
Moreover, gemcitabine interferes with ribonucleotide reductase’s function, a crucial enzyme for producing deoxyribonucleotides, the DNA building blocks. By obstructing ribonucleotide reductase, gemcitabine decreases the availability of deoxyribonucleotides, resulting in reduced DNA synthesis and ultimately cell death.
Gemcitabine has also been shown to stimulate specific signaling pathways that activate apoptosis, or programmed cell death, in cancer cells. This capability helps to eliminate cancer cells from the body and slow down the disease’s progression.
Gemcitabine’s potential to target DNA synthesis and repair and induce apoptosis makes it a highly effective chemotherapy drug for various cancers. However, the drug’s effectiveness is frequently limited by the development of drug resistance and its toxicity on healthy cells. Current research is aimed at discovering new ways to enhance gemcitabine’s effectiveness and reduce its adverse effects.
Gemcitabine’s mechanism of action involves disrupting DNA synthesis and repair and inducing apoptosis in cancer cells. While it has been shown to be highly effective in treating a variety of cancers, ongoing research is needed to overcome the limitations of the drug and improve its efficacy and safety.
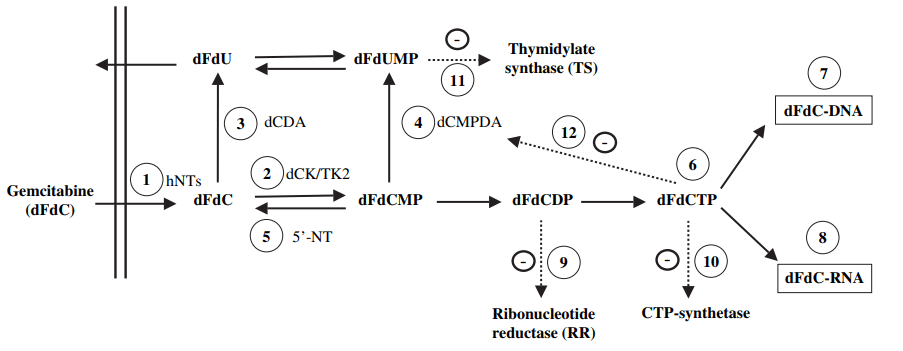
Figure 1 Metabolism, mechanisms of action and self-potentiation of gemcitabine[1]
III. Pharmacokinetics:
Gemcitabine is administered intravenously and is rapidly distributed throughout the body. It has a short half-life of approximately 30 minutes and is quickly metabolized by the liver and excreted in the urine. The drug is primarily eliminated unchanged through the kidneys, and its clearance is highly dependent on renal function. In patients with impaired renal function, gemcitabine clearance is decreased, leading to increased toxicity and the need for dose adjustments.[2]
Factors such as age, sex, and renal function can affect the pharmacokinetics of gemcitabine. In older patients and those with renal impairment, the clearance of the drug is reduced, leading to an increased risk of toxicity. Additionally, studies have shown that women may have a higher clearance of gemcitabine than men, which may impact the dosing and efficacy of the drug in this population.
IV. Clinical Efficacy:
Gemcitabine is an effective chemotherapy drug that has been extensively studied in numerous clinical trials for various cancer types. It can be administered as a single agent or in combination with other chemotherapy drugs or targeted agents. The response rates vary from 10-30% depending on the treatment regimen and cancer type, indicating that gemcitabine can be an effective treatment option for different cancers.[3]
One of the most significant studies of gemcitabine in pancreatic cancer is the CONKO-001 trial, a randomized controlled trial that compared gemcitabine with observation in patients who had undergone surgical resection for pancreatic cancer. The study found that gemcitabine improved disease-free survival and overall survival in these patients compared to observation alone, establishing gemcitabine as the standard of care for pancreatic cancer.
Gemcitabine has also been found to be effective in treating bladder cancer when used in combination with cisplatin. The MRC BL12 trial evaluated the combination of gemcitabine and cisplatin in patients with advanced bladder cancer and found that this regimen was associated with a higher response rate, longer progression-free survival, and overall survival compared to gemcitabine alone.[4.5]
In breast cancer, gemcitabine has been evaluated in combination with other chemotherapy drugs such as docetaxel, paclitaxel, and carboplatin. These studies have shown promising results, with response rates ranging from 33-68%, depending on the treatment regimen.
In lung cancer, gemcitabine has been studied in combination with other chemotherapy drugs such as cisplatin, vinorelbine, and paclitaxel. These studies have shown that gemcitabine-based regimens can be effective in both first-line and second-line treatment settings, with response rates ranging from 20-40%.[6]
Overall, gemcitabine has demonstrated clinical efficacy in the treatment of different cancer types, particularly pancreatic and bladder cancer. When used in combination with other chemotherapy drugs or targeted agents, gemcitabine has been shown to improve response rates, disease-free survival, progression-free survival, and overall survival in various cancer types. However, its effectiveness can be limited by the development of drug resistance and its toxicity on healthy cells. Ongoing research is focused on identifying new ways to enhance the effectiveness of gemcitabine and reduce its side effects.
V. Safety Profile:
Gemcitabine is generally well tolerated, but like all chemotherapy drugs, it can cause side effects. The most common adverse effects of gemcitabine include myelosuppression, nausea, vomiting, and fatigue. These side effects are usually mild to moderate in severity and can be managed with supportive care measures.[7,8]
Less common but more serious adverse effects of gemcitabine include pulmonary toxicity and infusion-related reactions. Pulmonary toxicity is characterized by shortness of breath, coughing, and fever, and can occur in up to 10% of patients treated with gemcitabine. Infusion-related reactions, such as fever, chills, and flushing, can occur during or shortly after the infusion of gemcitabine and can be managed with antihistamines and steroids.
Current research:
The paper titled “Recent advances and prospects in gemcitabine drug delivery systems” discusses the various drug delivery systems (DDS) that have been developed for the effective delivery of gemcitabine, an anticancer drug, to tumor sites. The authors highlight the limitations of conventional chemotherapy, such as poor solubility, non-specific distribution, and resistance to treatment, and how these issues can be addressed through the use of drug delivery systems.
The article reviews the different types of DDS for gemcitabine, such as liposomes, nanoparticles, dendrimers, and hydrogels. These systems aim to improve the pharmacokinetic properties of gemcitabine, including its solubility, bioavailability, and targeting efficiency, thereby enhancing its efficacy against cancer.[9]
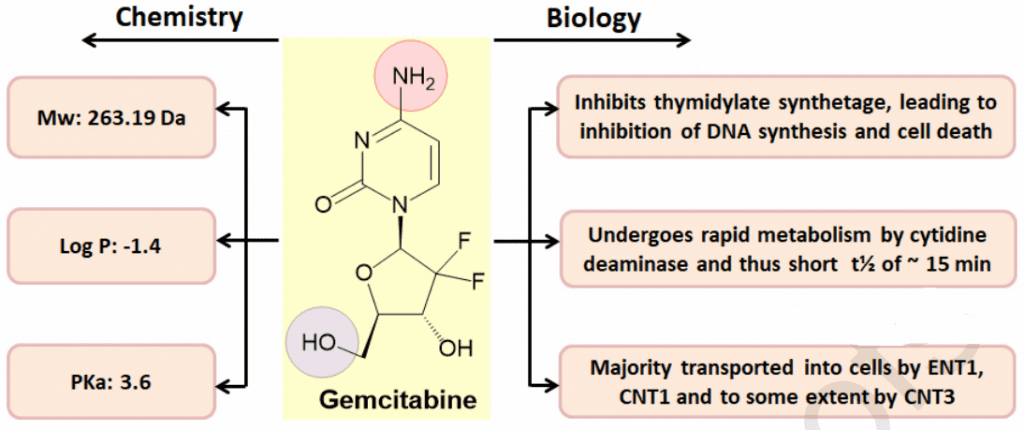
Figure 2 Chemical structure of gemcitabine with their chemical and biological properties[9]
The authors discuss the advantages and limitations of each type of DDS, highlighting the importance of choosing the appropriate DDS for a specific cancer type and patient population. For instance, liposomes have been extensively studied for gemcitabine delivery, as they offer high drug-loading capacity, prolonged circulation time, and the ability to target tumors through the enhanced permeability and retention (EPR) effect. However, liposomes may also cause toxicity issues and are prone to rapid clearance by the immune system.
Nanoparticles, on the other hand, have shown promise in gemcitabine delivery due to their small size, high surface area, and ability to penetrate tumor tissues. The authors discuss various types of nanoparticles, such as polymeric nanoparticles, gold nanoparticles, and silica nanoparticles, and their potential applications in gemcitabine delivery.
The paper also discusses the use of dendrimers, which are highly branched, nanoscale polymers with a well-defined structure. Dendrimers have been explored for the targeted delivery of gemcitabine due to their ability to encapsulate drugs and bind to cancer cells through surface modifications. However, their potential toxicity and high cost limit their clinical translation.
The authors also highlight the use of hydrogels, which are three-dimensional networks of hydrophilic polymers capable of encapsulating drugs and releasing them in a controlled manner. Hydrogels have shown promise in local delivery of gemcitabine, particularly for pancreatic cancer, as they can improve drug retention and minimize systemic toxicity.
Overall, the article concludes that the development of gemcitabine DDS has shown promising results in preclinical studies and clinical trials, highlighting the potential for improving cancer treatment outcomes. The authors emphasize the need for further research to optimize the design and formulation of DDS and to evaluate their safety and efficacy in clinical settings.
Summary
Gemcitabine is a chemotherapy drug that has been used for over two decades in the treatment of various types of cancer. It belongs to a class of drugs known as antimetabolites and works by interfering with the replication process of cancer cells. Gemcitabine is approved for the treatment of several cancer types, including pancreatic, breast, lung, bladder, and ovarian cancer.
Clinical studies have shown that gemcitabine is an effective chemotherapy drug, with response rates varying from 10-30% based on the treatment regimen and cancer type. In pancreatic cancer, gemcitabine has been found to enhance survival compared to other chemotherapy drugs and is currently the standard of care for this disease. The combination of gemcitabine with other drugs, such as cisplatin, has been shown to further improve survival rates in some cancer types.
Gemcitabine is typically administered intravenously, either as a single agent or in combination with other chemotherapy drugs or targeted agents. Its pharmacokinetics are influenced by factors such as age, sex, and renal function. While gemcitabine is generally well tolerated, it can cause side effects such as myelosuppression, nausea, and vomiting, as well as less common but more serious adverse effects such as pulmonary toxicity and infusion-related reactions.
Research is ongoing to discover new approaches to enhance the effectiveness of gemcitabine and reduce its toxicity. One area of focus is the development of drug delivery systems that can improve the pharmacokinetic and pharmacodynamic properties of gemcitabine, such as liposomes, nanoparticles, and microparticles. These delivery systems have the potential to increase the accumulation of gemcitabine in tumor cells, reduce its toxicity in healthy cells, and improve its therapeutic efficacy.
Reference:
[1] Mini, Nobili, Caciagli, Landini, & Mazzei. Cellular pharmacology of gemcitabine.
[4] Castellano, D., Paz-Ares, L., Pronk, L., Tomamira, M. V., Tabernero, J. M., Ciruelos, E., … & Cortes-Funes, H. (2000). A phase I/II clinical and pharmacologic study of dose-escalating and dose-sequencing administration of gemcitabine and folinic acid/fluorouracil in advanced unresectable pancreatic cancer. Ann Oncol, 11(suppl 4), 61.