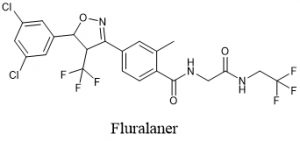
What is Fluralaner?
Abstract
Fluralaner is a new broad-spectrum insecticide with isoxazoline structure, which is an effective inhibitor of ligand-gated chloride channels in neurons. Compared with other similar insecticides, it has significant differences in molecular structure, action site, selectivity, and cross-resistance. According to the relevant literature, as a broad-spectrum insecticide, Fluralaner has good insecticidal activity against most agricultural pests in addition to animal parasites, which can provide more choices for the prevention of sanitary pests and agricultural pests.
What is Fluralaner
Fluralaner is a new broad-spectrum insecticide with isoxazoline structure, which is an effective inhibitor of ligand-gated chloride channels in neurons. Compared with other similar insecticides, it has significant differences in molecular structure, action site, selectivity, and cross-resistance. According to the relevant literature, as a broad-spectrum insecticide, Fluralaner has good insecticidal activity against most agricultural pests in addition to animal parasites, which can provide more choices for the prevention of sanitary pests and agricultural pests.
Agriculture as a source of food supply, for long-term, sustainable development, is inseparable from the support of pesticides. At present, there are many kinds of crop diseases, pests, and weeds, which are widely distributed, and the rate of variation and reproduction is fast. In the process of agricultural development, pests and diseases will endanger crop growth and even death, resulting in a certain degree of crop yield reduction, which has a significant impact on agricultural and economic development. At present, the use of chemical pesticides is still the main measure to control pests and diseases, which plays a crucial role in increasing crop yield, ensuring agricultural production safety, and ensuring food security. According to the natural loss rate of about 37 % published by FAO (Food and Agriculture Organization of the United Nations), the use of pesticides can save more than 500 billion yuan of economic losses every year. In the pesticide use structure, the proportion of insecticides is much higher than that of fungicides and herbicides.
The use of insecticides has a long history. The initial pest control originated from botanical drugs. People detoxify and kill pests by fumigation, soaking, and boiling. The species include bitter wood, tobacco, wild grass, rattan, pyrethrum, etc., and then later inorganic arsenic, fluorine, and mineral insecticides. Since the 1940 s, it has entered the era of organic synthetic insecticides. Almost every ten years, at least one new chemical structure category will be introduced, such as organochlorines, organophosphorus, carbamates, benzoylureas, nereistoxins, pyrethroids, neonicotinoids and ryanodine receptors. The structure of the world’s pesticide industry is also constantly changing. Traditional chemical pesticides such as organophosphorus (methamidophos, methyl parathion, monocrotophos, etc.), carbamate (carbofuran, methomyl, etc.), and organochlorine (DDT, toxaphene, etc.) have made great contributions to the prevention and control of pests and the increase of crop yield. However, with the frequent use of pesticide insecticides, there are also many problems such as high pollution and high residue of pesticide soil; agricultural pests have rapid variation and breeding speed, and serious resistance to insecticides; toxic to beneficial organisms such as bees; high carcinogenic hazards to human health, etc., has been restricted or prohibited by many countries. Therefore, how to develop products with high activity, high selectivity, low risk, and no residue has become the development direction of green pesticides.
As a lead compound, isoxazoline skeleton has high research and development value in crop protection. Isoxazoline insecticides are new y-aminobutyric acid-gated chloride channel blockers with novel action sites and high-efficiency broad-spectrum insecticidal activity. They are safe for non-target organisms and the environment. They have good insecticidal activity against highly resistant pests and can provide a good solution for pest resistance. At present, there are few varieties of isoxazoline pesticides with broad development prospects, which provides a good research direction and research ideas for the creation of green pesticides.
Targets and Mechanism of Isoxazoline Insecticides
After receiving external stimulation, neurons can quickly mediate the release of neurotransmitters. Neurotransmitters specifically act on Cys-loop Ligand-Gated Ion Channel to stimulate or inhibit the conduction of nerve impulses, further achieving signal transmission. Cysteinyl chloride channels include GABAC1, GluCl, and HisC1. GABAC1 is located in the postsynaptic membrane, which is an important part of GABAR. It can play a protective role in inhibiting nerve impulses by specifically binding to the inhibitory neurotransmitter GABA. On the contrary, the nerve impulses are in a state of destructive excitation. Scientists can develop drugs with different functions according to the interaction mode and mechanism between drugs and GABAR to meet the needs of use and production in the field of medicine and pesticide.
Molecular biology studies have shown that ionic GABA receptors play an important role in the central nervous system and peripheral nervous system of insects, and are membrane proteins directly involved in the transmembrane signal transmission of neurons and muscle cells. GABA is the main inhibitory neurotransmitter in the insect nervous system, and its inhibition is mediated by receptors. GABA is released into the synaptic cleft through the presynaptic membrane at the end of the nerve cell and then binds to the postsynaptic membrane receptor, opening the gated chloride channel. The change of membrane permeability leads to hyperpolarization, which in turn inhibits insect neural activity. Isogarazoline insecticides as NCAs act on chloride channels, inhibiting chloride influx so that it cannot penetrate the postsynaptic membrane, interfering with GABA transmembrane signal transmission, leading to insect nervous system disorders, and ultimately making insect nerve excitation uncontrollable and death.
Bioactivity of Flurellana
Fluralaner is a broad-spectrum, highly active, and highly selective compound, which has good biological activity against arthropods such as ticks, fleas, lice, hemiptera, diptera, lepidoptera and mite. Its mode of action is mainly stomach poison and contact killing.
As an important representative of insecticides targeting γ-aminobutyric acid receptors, the control effect of fipronil on agricultural pests was once recognized by farmers. Compared with fipronil, Fluralaner does not have cross-resistance and exhibits excellent activity against fipronil pests. The existing research shows that: Fluralaner has good activity with Lepidoptera (Centipedes dissimilis, Helicoverpa armigera, Spodoptera frugiperda), Hemiptera (Laodelphax striatellus, Vicia faba leafhopper), Thysanoptera (Frankliniella occidentalis), mites (Tetranychus urticae) and other agricultural pests, its mode of action is mainly stomach poison and contact killing. Researchers have found that the activity of Fluralaner against adult T. urticae is second only to abamectin but higher than pyridaben and hexythiazox. Notably, two other isoxazoline insecticides (fuxametamide and isocycloseram) are already in the process of commercialization. Japan’s Nissan Chemical Industry Co., Ltd. Fluconazole amide has been applied for registration in Korea fruit (apple) pest control; syngenta ‘s isocycloseram (development code SYN547407) has been provisionally approved by the International Organization for Standardization (ISO) technical committee for pesticide generic names.
The transport and metabolism of fluralaner in organisms
Fluralaner has the characteristics of high apparent volume of distribution, low clearance rate and long half-life. The long persistence of Fluralaner in organisms is related to the way drugs are metabolized in the body. In mammals, Fluralaner is mainly excreted through the liver but is almost not detected in the kidney excreta. For mosquitoes, S, S, S-Tributyl phosphorotrithioate (DEF) can effectively increase the activity of fluralaner (3.8-8 fold), while piperomyl butoxide (PBO) and diethyl maleate (DEM) have no significant synergistic effect. However, piperonyl butoxide significantly increased the activity of Fluralaner against S.litura larvae (1.55-1.99 fold). In a word, the specific metabolism of Fluralaner in organisms needs more exploration.
Effects of Fluralaner on non-target organisms
Effects of Fluralaner on vector /economic insects
Research on the effects of pesticides on vector/economic insects such as bees is receiving increasing attention. Its selectivity to this kind of insect has an important guiding role in its application scope and market promotion. Existing studies have shown that Fluralaner is highly toxic to bees (LD50 of 48 h acute oral and contact toxicity is 0.13 ug/bee), indicating that Fluralaner must be away from bee colonies and nectar plants when applied in agricultural production; however, for greenhouses, greenhouses and other controllable planting areas, self-pollinated crop areas and non-flowering plants (such as tea plants), there is still a huge space for use in Fluralaner.
Effects of Fluralaner on aquatic organisms
After the pesticide is sprayed on the surface of the plant, part of it is volatilized or drifted to other places, and the other part enters the soil and rivers through the action of rainwater erosion, leaching, and surface runoff. In particular, the impact of pesticide residues on aquatic organisms after entering the water body restricts its application area and application method to a certain extent. The results showed that the toxicity of Fluralaner to Brachydanio rerio and Cyprinus carpio were low (LC50 > 10 mg/L, 96 h) and toxic (LC50 = 2 mg/L, 96 h), respectively. The toxicity of Fluralaner to Daphnia magna was high (EC50 = 0.0001-0.01 mg/L, 48 h), and the toxicity of Fluralaner to Pseudokichneriela subcapiata was low ( EC50 > 10 mg/L, 72 h). However, the effects of Fluralaner on aquatic organisms such as shrimp, crabs, and shell animals have not been reported.
Summary
As a representative of isoxazole insecticides, the research depth, marketing mode, and current situation of fluralaner can provide strong reference value for the development of other similar insecticides. On the one hand, as a new generation of pesticides acting on the γ-aminobutyric acid receptor, the unique mode of action of fluralaner deserves our attention; on the other hand, from the perspective of target and drug synthesis, clarifying its specific target and mechanism of action will play an important guiding role in transforming its active groups and developing new similar compounds.