5 Milestone New Drugs That Changed the World
Abstract
In the last century, the endeavors of chemists to create novel pharmaceuticals have emerged as a fundamental pillar of contemporary medicine. In a commemorative edition marking its centenary, C&EN, an affiliate of the American Chemical Society, retrospectively examined five pivotal breakthrough drugs from the past century. This insightful article underscored how these five compounds not only transformed medical protocols but also sparked revolutionary shifts in the scientific methodologies of drug exploration, subsequently leaving an indelible impact on societal norms.
Penicillin: The Antibiotic That Saved Millions of Lives
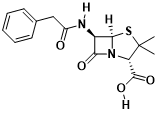
Fig. 1 penicillin molecular structure
Penicillin stands as the pioneering antibiotic effective against an extensive spectrum of bacterial infections, ushering in a new era of treating once-deadly ailments. In 1928, Sir Alexander Fleming’s groundbreaking observation revealed bacterial growth inhibition near mold in a Petri dish. Building upon this revelation, Oxford University researchers Howard Florey and Ernst Chain, a decade later, confirmed penicillin’s bactericidal prowess in mice and humans. They subsequently honed a systematic synthesis method. The culmination of their efforts materialized in 1945 when the trio was honored with the Nobel Prize in Physiology or Medicine for their seminal contributions to penicillin’s discovery.
Presently, penicillin remains among the globally prevalent antibiotics, retaining its status as a covalent drug adept at establishing irreversible covalent bonds with targets, thereby effecting permanent functional alterations. The notion of purposefully devising covalent drugs has garnered escalating interest in contemporary times. This fascination has yielded not only groundbreaking therapeutic breakthroughs but also ushered in innovative approaches for engaging previously elusive targets, opening avenues for targeting traditionally challenging entities.
Chlorpromazine: One of the first drugs to directly treat psychological disorders
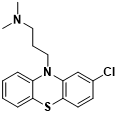
Fig. 2 Chlorpromazine molecular structure
Initially formulated to aid patients in achieving emotional equilibrium during surgical anesthesia, chlorpromazine found its origins. In 1952, Dr. Henri Laborit, a French physician, embarked on a trial to assess the impact of this drug, coupled with additional therapies, on a 24-year-old individual grappling with psychosis. Remarkably, over a span of three weeks, this intervention facilitated such significant improvement that the patient’s condition ameliorated to the extent of warranting discharge from the medical facility.
The swift endorsement of chlorpromazine for individuals experiencing psychotic episodes propelled its adoption, culminating in its approval by the US FDA in 1954. This marked its inclusion among the initial wave of antipsychotics. Its introduction signaled a pivotal juncture in the management of mental disorders through pharmacological means, heralding the era of psychopharmacology and forever transforming the landscape of psychological practice.
Oral Contraceptives: The Pill That Changed Society
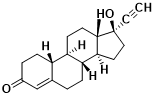
Fig. 3 Norethindrone molecular structure
Since the 1920s, researchers have been aware that administering reproductive hormones like progesterone through animal injections can avert pregnancy. However, employing progesterone as a direct pharmaceutical has encountered numerous obstacles. Its efficacy in humans necessitates either injection or consumption in substantial quantities.
In 1951, chemists Carl Djerassi, Luis Miramontes, and George Rosenkranz achieved a significant milestone by creating the inaugural progesterone analog, norethindrone. This compound displayed oral efficacy at minimal dosages, thereby initiating the trajectory toward oral contraceptive development.
Subsequently, in 1957, the US FDA approved the inaugural amalgamation therapy involving norethindrone and mestranol to address menstrual irregularities. By 1960, it received its first endorsement as an oral contraceptive. This pivotal innovation dramatically reshaped society by empowering women with the ability to strategize their family planning.
Antiviral Therapy: Turning a Deadly Disease into a Manageable Chronic Disease
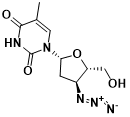
Fig. 4 Molecular structure of Zidovudine
During the 1980s, the emergence of AIDS brought about profound dread due to its lethal impact. Yet, thanks to scientists’ unwavering dedication, the swift identification of the HIV virus responsible for AIDS catalyzed the development of antiviral interventions. A pivotal moment arose in 1987 when the US FDA sanctioned the inaugural antiviral therapy, zidovudine (also known as zidovudine). This landmark development marked humanity’s initial foray into combating HIV. By curbing viral replication through the inhibition of viral reverse transcriptase, this therapy emerged as the first line of defense against the virus.
Nonetheless, relying on a single drug for HIV treatment quickly leads to drug-resistant strains of the virus. In the mid-1990s, Professor He Dayi and his team at the Rockefeller University AIDS Research Institute introduced what later became renowned as “cocktail therapy.” Their groundbreaking insight revealed that administering a combination of 3-4 distinct drugs simultaneously could potentially outpace the mutation rate of the HIV, effectively immobilizing it. The inaugural protease inhibitor, saquinavir, gained approval from the US FDA in late 1995. In 1996, Professor He Dayi’s team made a groundbreaking discovery: the virus levels in patients undergoing cocktail therapy plummeted to an undetectable extent, sparking widespread enthusiasm. This landmark achievement led to the adoption of Highly Active Antiretroviral Therapy (HAART) as the standard AIDS treatment in the subsequent year. Notably, this catalyzed a remarkable 47% decline in AIDS-related mortality, a nearly halved death rate, illustrating the transformative power of this approach.
In the contemporary landscape, with a burgeoning array of antiviral medications, individuals living with HIV can anticipate a quality of life akin to that of the general population, provided they adhere to prescribed drug regimens. These medications not only extend patients’ lifespans but also serve as a preventive measure against HIV transmission, particularly within high-risk communities.
Imatinib: Opening a new chapter in targeted cancer therapy
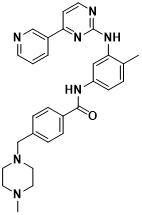
Fig. 5 Molecular structure of imatinib
Imatinib, commercially known as Gleevec, gained its initial FDA approval in 2001 as a remedy for chronic myeloid leukemia (CML). It marked a pioneering stride into precision therapy, tailored to target explicit genetic anomalies contributing to cancer, thereby inaugurating the era of precise cancer treatment. Initially, developers hesitated to focus on protein kinases due to the multitude of these enzymes involved in vital physiological functions; imprecise targeting could evoke toxicity. In the late 1980s, Ciba-Geigy scientists embarked on a quest to pinpoint protein kinase inhibitors. Through a series of strategic refinements, imatinib emerged with remarkable specificity, adeptly inhibiting the growth of cells expressing the BCR-Abl protein accountable for CML.
In the realm of clinical application, this medication has exhibited a remarkable impact, elevating the 5-year survival rate of patients with CML from a former 30% to an approximate 90%.
Note: The products we provide are for scientific research purposes only, not for clinical use.