A New Target for Cancer Immunotherapy-CCR2
CCL2-CCR2 axis refers to the interaction between the chemokine ligand CCL2 (also known as monocyte chemoattractant protein-1 or MCP-1) and its receptor CCR2 (C-C chemokine receptor type 2). This axis plays an important role in the recruitment and activation of immune cells, particularly monocytes and macrophages, in response to inflammation or injury.
The role of CCL2-CCR2 axis in tumorigenesis and development
Regulation of CCL2 gene expression
CCL2, also known as monocyte chemoattractant protein-1 (MCP-1), is a member of the CC subfamily of chemokines. It is a small cytokine that plays an important role in recruiting immune cells, including monocytes, macrophages and T lymphocytes, to sites of inflammation and injury. The human CCL2 gene is located on chromosome 17 and contains three exons and two introns. The precursor form of CCL2 is secreted, and the mature protein consists of 76 amino acid residues.
CCL2 is capable of binding to both CCR2 and CCR4 chemokine receptors, but it exhibits a greater affinity for CCR2, a G protein-coupled receptor present on the surface of various cell types, including basophils, monocytes, natural killer cells, and T cells. Despite the fact that the binding between CCL2 and CCR2 is not highly specific, research indicates that CCL2 primarily exerts its biological effects by binding to CCR2.
CCL2 can be produced by a variety of activated cells, such as fibroblasts, endothelial cells, lymphocytes, and macrophages. In addition, some tumor cells, including breast cancer, lung cancer, prostate cancer, and colorectal cancer, can also produce CCL2. Transcription factors such as NF-κB, Sp1, AP-1, and p53 are involved in the regulation of CCL2 basal expression or inflammatory stimulation expression. Cytokines such as TNFα, LPS, IL-1, IL-6, and TGF-β in the tumor microenvironment can stimulate tumor cells to significantly promote the expression of CCL2.
Recent studies have shown that in addition to these known classical transcription factors and activation pathways, there are some important non-classical pathways involved in the regulation of CCL2. Mammalian target of rapamycin complex 1 (mTORC1) is a protein kinase that can be activated by insulin. The forkhead box K1 (FOXK1) transcription factor belongs to the forkhead box (Fox) transcription factor family. The Notch signaling pathway is highly conserved in evolution and regulates the development of cells, tissues, and organs. Intracellular NICD is the activated form of the Notch receptor protein. It has been reported that two non-classical pathways, mTORC1-FOXK1 and Notch1-NICD1, are involved in the regulation of CCL2, thereby promoting the recruitment and infiltration of tumor-associated macrophages (TAMs).
Tumor-associated macrophages (TAMs) are a type of immune cell that infiltrates the tumor microenvironment and promote tumor cell growth, invasion, and metastasis. TAMs share many characteristics with M2 macrophages, which are known to mature under the influence of the tumor microenvironment. In Japan, studies have revealed that mTORC1 activation induces the dephosphorylation of downstream transcription factor FOXK1, which stimulates the expression of CCL2. Additionally, nude mouse animal models have demonstrated that the anti-tumor drug rapamycin can reduce the infiltration of TAMs in the tumor microenvironment.
In addition, the F-Box and WD40 domain protein 7 (FBXW7) has been shown to mediate the degradation of c-myc, Notch, cyclin E, c-Jun, and KLF5. Researchers knocked out Fbxw7 in mouse bone marrow mesenchymal stem cells and found that it could induce the accumulation of Notch protein and then transcriptionally activate the CCL2 gene, leading to the recruitment of macrophages.
CCL2-CCR2 axis promotes tumor metastasis
Tumor metastasis is a defining feature of malignant tumors, and the majority of tumor patients die due to tumor metastasis. In 1889, the British doctor Paget put forward the famous “seed and soil theory” after analyzing the data of breast cancer patients. Recent studies have provided new evidence that tumor cells not only adapt to the new microenvironment during metastasis but also transform the microenvironment into a pre-metastatic state. The tumor microenvironment comprises fibroblasts, immune and inflammatory cells, and glial cells surrounding tumor cells. The interaction between the tumor microenvironment and tumor cells is a key area of research in the field of tumor development and metastasis. As one of the most important and extensively studied chemokines in the tumor microenvironment, CCL2 promotes tumor cell growth and survival through autocrine or paracrine signaling, participates in the regulation of tumor immune tolerance, induces tumor angiogenesis, and facilitates tumor invasion and metastasis. Many studies have shown that elevated CCL2 levels are present in tissues or serum of patients with breast cancer, prostate cancer, liver cancer, colon cancer, pancreatic cancer, gastric cancer, and lung cancer, and are closely associated with tumor progression, invasion, metastasis, and prognosis. Currently, there are three main mechanisms by which CCL2 promotes tumor metastasis.
- CCL2-CCR2 axis promotes tumor metastasis directly or by recruiting tumor-associated macrophages ( TAM )
- CCL2-CCR2 axis promotes tumor metastasis by activating tumor-believing signaling pathway
- CCL2-CCR2 axis promotes tumor metastasis by inducing EMT in tumor cells
The inhibitory effect of CCR2 on adenocarcinoma
Chemokine receptor CCR2 exists on the surface of immune cells and can guide immune cells to inflammation and tumor sites. Breast cancer cells also express CCR2, but the specific function is unclear.
The researchers found that in the breast cancer mouse model, the deletion of the gene encoding CCR2 in cancer cells significantly reduced the growth rate of the tumor, and the survival time of the mice was prolonged by about twice. They further studied the biological processes behind this difference. The results showed that cancer cells expressing CCR2 produce an immunosuppressive microenvironment. The surface level of class I MHC ( responsible for presenting antigen proteins to killer T cells ) of CCR2-negative cancer cells is higher, and the level of PD-L1 is lower.
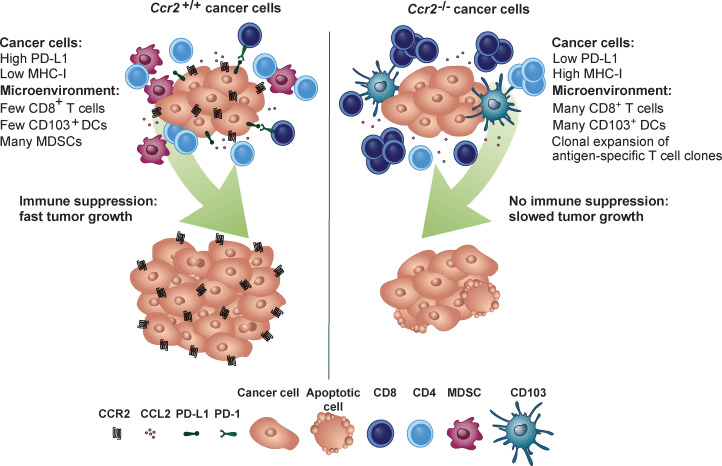
Figure 1[1] Graphical abstract
CCR2 signaling seems to achieve immunosuppression by disrupting the maturation of dendritic cells. Dendritic cells are the most important antigen-presenting cells and are essential for the initiation of immune responses. Compared with cancer cells expressing CCR2, mature dendritic cells and activated CD8 + killer T cells are more invasive in tumors generated by cells that do not express CCR2.
This new finding provides a theoretical basis for the hypothesis that blocking the CCR2 signal transduction of breast cancer cells helps the body ‘s own immune system to resist cancer.
According to Qian et al.’s findings, CCL2 recruits CCR2+ inflammatory monocytes to promote the metastasis of mouse breast cancer cell Met-1 (PyMT-induced). The lung metastasis of Met-1 cells was significantly inhibited by human or mouse-derived CCL2 antibody. Bonapace et al. also reported that CCL2 antibody (from R&D company) blocked CCL2 in mouse breast cancer cell 4T1.2, and reduced monocyte release from bone marrow, macrophage infiltration, vascular penetration, and formation of lung metastases. However, discontinuation of anti-CCL2 therapy accelerated lung metastasis, angiogenesis, and death in mice. After 10 days of discontinuation, CCL2 levels rebounded rapidly and increased significantly, causing bone marrow to release a large number of monocytes into the blood, which were recruited by CCL2 to further differentiate into macrophages. Vascular growth factor (VEGF-A) and interleukin-6 (IL-6) produced by macrophages may be involved in mediating this process. The anti-metastasis effect of anti-CCL2 antibody was still observed when it was continued. After stopping anti-CCL2 treatment, anti-VEGF-A treatment could reverse the rapid metastasis of the tumor and make the tumor progress at a normal rate. These findings suggest that targeting the tumor microenvironment is crucial for successfully inhibiting tumor cell metastasis, and anti-CCL2 treatment as a single drug therapy for tumor metastasis should be carefully considered.
CCR2 inhibitor CCX872
Pancreatic cancer is a highly lethal disease, with over 337,000 cases diagnosed worldwide each year and a 5-year survival rate of only 7%. Recently, ChemoCentryx released the survival data from their Phase Ib clinical trial of CCX872, a CCR2 inhibitor, in pancreatic cancer patients. The Phase I clinical trials of CCX872 in healthy volunteers had previously demonstrated the drug’s tolerability and suitability for long-term administration. The Phase Ib clinical trial was a multicenter open-label study that enrolled 50 patients with advanced unresectable pancreatic cancer (76% of patients had metastasis). In this trial, CCX872 was combined with FOLFIRINOX, a chemotherapy regimen consisting of 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin. All patients received FOLFIRINOX every two weeks for up to 12 cycles and daily doses of CCX872 for 12 weeks. The overall survival rate at 18 months was 29%, which represents a significant improvement compared to the 18.2% survival rate observed in the FOLFIRINOX trial alone.
Conclusion and prospect
In conclusion, CCL2 is a crucial factor in the promotion of tumor growth, invasion, and metastasis. While the CCR2 inhibitor CCX872 is generally well-tolerated, current clinical trial outcomes have been unsatisfactory due to insufficient blockage of the CCL2-CCR2 axis over a prolonged duration. We anticipate that a better understanding of the mechanisms behind treatment strategies targeting the CCL2-CCR2 axis could lead to innovative approaches to anti-tumor-associated macrophage therapy and the inhibition of tumor metastasis, thereby benefiting a greater number of cancer patients.
References
- Fein MR, He XY, Almeida AS, Bružas E, Pommier A, Yan R, Eberhardt A, Fearon DT, Van Aelst L, Wilkinson JE, Dos Santos CO, Egeblad M. Cancer cell CCR2 orchestrates suppression of the adaptive immune response. J Exp Med. 2020 Oct 5;217(10):e20181551. doi: 10.1084/jem.20181551. PMID: 32667673; PMCID: PMC7537399.
- Hao Q, Vadgama JV, Wang P. CCL2/CCR2 signaling in cancer pathogenesis. Cell Commun Signal. 2020 May 29;18(1):82. doi: 10.1186/s12964-020-00589-8. PMID: 32471499; PMCID: PMC7257158.
- Xu M, Wang Y, Xia R, Wei Y, Wei X. Role of the CCL2-CCR2 signalling axis in cancer: Mechanisms and therapeutic targeting. Cell Prolif. 2021 Oct;54(10):e13115. doi: 10.1111/cpr.13115. Epub 2021 Aug 31. PMID: 34464477; PMCID: PMC8488570.
- Fei L, Ren X, Yu H, Zhan Y. Targeting the CCL2/CCR2 Axis in Cancer Immunotherapy: One Stone, Three Birds? Front Immunol. 2021 Nov 3;12:771210. doi: 10.3389/fimmu.2021.771210. PMID: 34804061; PMCID: PMC8596464.
- Kadomoto S, Izumi K, Mizokami A. Roles of CCL2-CCR2 Axis in the Tumor Microenvironment. Int J Mol Sci. 2021 Aug 8;22(16):8530. doi: 10.3390/ijms22168530. PMID: 34445235; PMCID: PMC8395188.
- Fantuzzi L, Tagliamonte M, Gauzzi MC, Lopalco L. Dual CCR5/CCR2 targeting: opportunities for the cure of complex disorders. Cell Mol Life Sci. 2019 Dec;76(24):4869-4886. doi: 10.1007/s00018-019-03255-6. Epub 2019 Aug 3. PMID: 31377844; PMCID: PMC6892368.