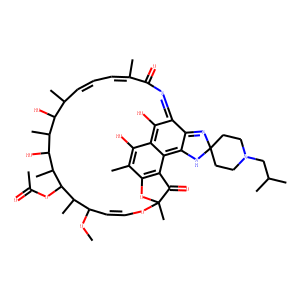
| IUPAC Name | [(7S,9E,11S,12R,13S,14R,15R,16R,17S,18S,19E,21Z)-2,15,17,32-tetrahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-1'-(2-methylpropyl)-6,23-dioxospiro[8,33-dioxa-24,27,29-triazapentacyclo[23.6.1.14,7.05,31.026,30]tritriaconta-1(32),2,4,9,19,21,24,26,30-nonaene-28,4'-piperidine]-13-yl] acetate |
| Reference | <p style=”/line-height:25px/”>
<br />
[1]. Review Article: Rifabutin in the Treatment of Refractory Helicobacter pylori Infection J. P. Gisbert, X. Calvet Issue Alimentary Pharmacology & Therapeutics Volume 35, Issue 2, pages 209-221, January 2012 Abstract Background Even with the current most effective treatment regimens, a relevant proportion of patients will fail to eradicate Helicobacter pylori infection. Aim To evaluate the role of rifabutin in the treatment of H. pylori infection. Methods Bibliographical searches were performed in MEDLINE. Data on the efficacy of rifabutin-containing regimens on H. pylori eradication were combined and meta-analysed using the generic inverse variance method. Results Rifabutin shows good in vitro activity against H. pylori. Mean H. pylori rifabutin resistance rate (calculated from 11 studies including 2982 patients) was 1.3% (95% confidence interval = 0.9-1.7%). When only studies including patients na?ve to H. pylori eradication treatment were considered, this figure was even lower (0.6%). On the other hand, higher values of rifabutin resistance were calculated (1.59%) when only post-treatment patients were considered. …<br />
[2]. Vourvahis M, Davis J, Wang R, Layton G, Choo HW, Chong CL, Tawadrous M. Effect of rifampin and rifabutin on the pharmacokinetics of lersivirine and effect of lersivirine on the pharmacokinetics of rifabutin and 25-o-desacetyl-rifabutin in healthy subjects. Antimicrob Agents Chemother. 2012 Aug;56(8):4303-9. Abstract Lersivirine is a nonnucleoside reverse transcriptase inhibitor (NNRTI) with a unique resistance profile exhibiting potent antiviral activity against wild-type HIV and several clinically relevant NNRTI-resistant strains. Lersivirine, a weak inducer of the cytochrome P450 (CYP) enzyme CYP3A4, is metabolized by CYP3A4 and UDP glucuronosyltransferase 2B7 (UGT2B7). Two open, randomized, two-way (study 1; study A5271008) or three-way (study 2; study A5271043) crossover phase I studies were carried out under steady-state conditions in healthy subjects. Study 1 (n = 17) investigated the effect of oral rifampin on the pharmacokinetics (PKs) of lersivirine. Study 2 (n = 18) investigated the effect of oral rifabutin on the PKs of lersivirine and the effect of lersivirine on the PKs of rifabutin and its active metabolite, 25-O-desacetyl-rifabutin. Coadministration with rifampin decreased the profile of the lersivirine area under the plasma concentration-time curve from time zero to 24 h postdose (AUC(24)), maximum plasma concentration (C(max)), and plasma concentration observed at 24 h postdose (C(24)) by 85% (90% confidence interval [CI], 83, 87), 83% (90% CI, 79, 85), and 92% (90% CI, 89, 94), respectively, versus the values for lersivirine alone. Coadministration with rifabutin decreased the lersivirine AUC(24), C(max), and C(24) by 34% (90% CI, 29, 39), 25% (90% CI, 16, 33), and 58% (90% CI, 52, 64), respectively, compared with the values for lersivirine alone. Neither the rifabutin concentration profile nor overall exposure was affected following coadministration with lersivirine. Lersivirine and rifabutin reduced the 25-O-desacetyl-rifabutin AUC(24) by 27% (90% CI, 21, 32) and C(max) by 27% (90% CI, 19, 34). Lersivirine should not be coadministered with rifampin, which is a potent inducer of CYP3A4, UGT2B7, and P-glycoprotein activity and thus substantially lowers lersivirine exposure. No dose adjustment of rifabutin is necessary in the presence of lersivirine; an upward dose adjustment of lersivirine may be warranted when it is coadministered with rifabutin.<br />
[3]. Gisbert JP, Castro-Fernandez M, Perez-Aisa A, Cosme A, Molina-Infante J, Rodrigo L, Modolell I, Cabriada JL, Gisbert JL, Lamas E, Marcos E, Calvet X. Fourth-line rescue therapy with rifabutin in patients with three Helicobacter pylori eradication failures. Aliment Pharmacol Ther. 2012 Apr;35(8):941-7. doi: 10.1111/j.1365-2036.2012.05053.x. Abstract BACKGROUND: In some cases, Helicobacter pylori infection persists even after three eradication treatments.AIM: To evaluate the efficacy of an empirical fourth-line rescue regimen with rifabutin in patients with three eradication failures.METHODS: Design: Multicentre, prospective study. Patients: In whom the following three treatments had consecutively failed: first (PPI + clarithromycin + amoxicillin); second (PPI + bismuth + tetracycline + metronidazole); third (PPI + amoxicillin + levofloxacin). Intervention: A fourth regimen with rifabutin (150 mg b.d.), amoxicillin (1 g b.d.) and a PPI (standard dose b.d.) was prescribed for 10 days. Outcome: Eradication was confirmed by (13) C-urea breath test 4-8 weeks after therapy. Compliance and tolerance: Compliance was determined through questioning and recovery of empty medication envelopes. Adverse effects were evaluated using a questionnaire.RESULTS: One-hundred patients (mean age 50 years, 39% men, 31% peptic ulcer/69% functional dyspepsia) were included. Eight patients did not take the medication correctly (in six cases due to adverse effects). Per-protocol and intention-to-treat eradication rates were 52% (95% CI = 41-63%) and 50% (40-60%). Adverse effects were reported in 30 (30%) patients: nausea/vomiting (13 patients), asthenia/anorexia (8), abdominal pain (7), diarrhoea (5), fever (4), metallic taste (4), myalgia (4), hypertransaminasemia (2), leucopenia (<br />
[4]. Gisbert JP, Calvet X. Review article: rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther. 2012 Jan;35(2):209-21. doi: 10.1111/j.1365-2036.2011.04937.x. Abstract BACKGROUND: Even with the current most effective treatment regimens, a relevant proportion of patients will fail to eradicate Helicobacter pylori infection.AIM: To evaluate the role of rifabutin in the treatment of H. pylori infection.METHODS: Bibliographical searches were performed in MEDLINE. Data on the efficacy of rifabutin-containing regimens on H. pylori eradication were combined and meta-analysed using the generic inverse variance method.RESULTS: Rifabutin shows good in vitro activity against H. pylori. Mean H. pylori rifabutin resistance rate (calculated from 11 studies including 2982 patients) was 1.3% (95% confidence interval = 0.9-1.7%). When only studies including patients na?ve to H. pylori eradication treatment were considered, this figure was even lower (0.6%). On the other hand, higher values of rifabutin resistance were calculated (1.59%) when only post-treatment patients were considered. Overall, mean H. pylori eradication rate (intention-to-treat analysis) with rifabutin-containing regimens (1008 patients) was 73% (67-79%). Respective cure rates for second-line (223 patients), third-line (342 patients) and fourth/fifth-line (95 patients) rifabutin therapies were 79% (67-92%), 66% (55-77%) and 70% (60-79%) respectively. For treating H. pylori infection, almost all studies have administered rifabutin 300 mg/day; this dose seems to be more effective than 150 mg/day. The ideal length of treatment remains unclear, but 10- to 12-day regimens are generally recommended. The mean rate of adverse effects was 22% (19-25%). Myelotoxicity is the most significant, although this complication was rare. Until now, all patients have recovered of leucopenia uneventfully in a few days, and there have been no reports of infection or other adverse outcomes related to it.CONCLUSION: Rifabutin-containing rescue therapy constitutes an encouraging strategy after multiple (usually three) previous eradication failures with key antibiotics such as amoxicillin, clarithromycin, metronidazole, tetracycline and levofloxacin.<br />
[5]. Horne DJ, Spitters C, Narita M. Experience with rifabutin replacing rifampin in the treatment of tuberculosis. Int J Tuberc Lung Dis. 2011 Nov;15(11):1485-9, i. Abstract SETTING: The use of a rifamycin in anti-tuberculosis treatment regimens is crucial for shortening treatment and achieving favorable outcomes. Rifampin (RMP) is the recommended rifamycin, although adverse effects (AEs) may require its discontinuation. The use of rifabutin (RFB), a rifamycin with activity against Mycobacterium tuberculosis, in patients with an RMP-related AE has not been well studied.OBJECTIVE: To review our experience with RFB in tuberculosis (TB) treatment.METHODS: We included TB patients who received RFB in their treatment regimens from 2003 to 2009. We evaluated the indications for RFB and, if applicable, the likelihood that RMP caused an AE. We identified RMPrelated AEs associated with RFB intolerance.RESULTS: One hundred subjects were included. The indications for RFB use were RMP-related AE (57%), con- current antiretroviral therapy (21%), potential/actual interaction with other medications (14%), and as part of an alternative regimen in liver disease (8%). Nineteen patients experienced an AE while taking RFB. Among patients with a prior RMP-related AE, 80% of whom were successfully treated with RFB, only a dermatologic AE was associated with subsequent RFB intolerance.CONCLUSIONS: Our study suggests that RFB is well tolerated by patients who develop RMP-related AEs. There may be an increased risk for RFB-related AE in patients who experienced RMP-related dermatologic events.</p>
|