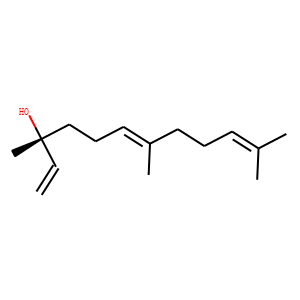
| Reference | </br>1: Saito AY, Sussmann RA, Kimura EA, Cassera MB, Katzin AM. Quantification of nerolidol in mouse plasma using gas chromatography-mass spectrometry. J Pharm Biomed Anal. 2015;111:100-3. doi: 10.1016/j.jpba.2015.03.030. Epub 2015 Apr 3. PubMed PMID: 25880240; PubMed Central PMCID: PMC4673400.</br>2: Chan WK, Tan LT, Chan KG, Lee LH, Goh BH. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules. 2016 Apr 28;21(5). pii: E529. doi: 10.3390/molecules21050529. Review. PubMed PMID: 27136520.</br>3: Fonsêca DV, Salgado PR, de Carvalho FL, Salvadori MG, Penha AR, Leite FC, Borges CJ, Piuvezam MR, Pordeus LC, Sousa DP, Almeida RN. Nerolidol exhibits antinociceptive and anti-inflammatory activity: involvement of the GABAergic system and proinflammatory cytokines. Fundam Clin Pharmacol. 2016 Feb;30(1):14-22. doi: 10.1111/fcp.12166. Epub 2015 Nov 22. PubMed PMID: 26791997.</br>4: Ma C, Qu Y, Zhang Y, Qiu B, Wang Y, Chen X. Determination of nerolidol in teas using headspace solid phase microextraction-gas chromatography. Food Chem. 2014;152:285-90. doi: 10.1016/j.foodchem.2013.11.010. Epub 2013 Nov 28. PubMed PMID: 24444938.</br>5: Silva MP, Oliveira GL, de Carvalho RB, de Sousa DP, Freitas RM, Pinto PL, de Moraes J. Antischistosomal activity of the terpene nerolidol. Molecules. 2014 Mar 24;19(3):3793-803. doi: 10.3390/molecules19033793. PubMed PMID: 24662089.</br>6: de Assis Lage TC, Montanari RM, Fernandes SA, de Oliveira Monteiro CM, de Oliveira Souza Senra T, Zeringota V, da Silva Matos R, Daemon E. Chemical composition and acaricidal activity of the essential oil of Baccharis dracunculifolia De Candole (1836) and its constituents nerolidol and limonene on larvae and engorged females of Rhipicephalus microplus (Acari: Ixodidae). Exp Parasitol. 2015 Jan;148:24-9. doi: 10.1016/j.exppara.2014.10.011. Epub 2014 Oct 24. PubMed PMID: 25448290.</br>7: Krist S, Banovac D, Tabanca N, Wedge DE, Gochev VK, Wanner J, Schmidt E, Jirovetz L. Antimicrobial activity of nerolidol and its derivatives against airborne microbes and further biological activities. Nat Prod Commun. 2015 Jan;10(1):143-8. PubMed PMID: 25920237.</br>8: Nogueira Neto JD, de Almeida AA, da Silva Oliveira J, Dos Santos PS, de Sousa DP, de Freitas RM. Antioxidant effects of nerolidol in mice hippocampus after open field test. Neurochem Res. 2013 Sep;38(9):1861-70. doi: 10.1007/s11064-013-1092-2. Epub 2013 Jun 14. PubMed PMID: 23765368.</br>9: Saito AY, Marin Rodriguez AA, Menchaca Vega DS, Sussmann RA, Kimura EA, Katzin AM. Antimalarial activity of the terpene nerolidol. Int J Antimicrob Agents. 2016 Dec;48(6):641-646. doi: 10.1016/j.ijantimicag.2016.08.017. Epub 2016 Sep 30. PubMed PMID: 27742206.</br>10: Javed H, Azimullah S, Abul Khair SB, Ojha S, Haque ME. Neuroprotective effect of nerolidol against neuroinflammation and oxidative stress induced by rotenone. BMC Neurosci. 2016 Aug 22;17(1):58. doi: 10.1186/s12868-016-0293-4. PubMed PMID: 27549180; PubMed Central PMCID: PMC4994214.</br>11: Goel RK, Kaur D, Pahwa P. Assessment of anxiolytic effect of nerolidol in mice. Indian J Pharmacol. 2016 Jul-Aug;48(4):450-452. PubMed PMID: 27756960; PubMed Central PMCID: PMC4980937.</br>12: Sperotto AR, Moura DJ, Péres VF, Damasceno FC, Caramão EB, Henriques JA, Saffi J. Cytotoxic mechanism of Piper gaudichaudianum Kunth essential oil and its major compound nerolidol. Food Chem Toxicol. 2013 Jul;57:57-68. doi: 10.1016/j.fct.2013.03.013. Epub 2013 Mar 20. PubMed PMID: 23523831.</br>13: Le Thanh C, Chauhan KR. Simple and short synthesis of trans-(R)-nerolidol, a pheromone component of fruit spotting bug. Nat Prod Commun. 2014 Mar;9(3):297-8. PubMed PMID: 24689198.</br>14: Baldissera MD, Grando TH, Souza CF, Cossetin LF, Sagrillo MR, Nascimento K, da Silva AP, Dalla Lana DF, Da Silva AS, Stefani LM, Monteiro SG. Nerolidol nanospheres increases its trypanocidal efficacy against Trypanosoma evansi: New approach against diminazene aceturate resistance and toxicity. Exp Parasitol. 2016 Jul;166:144-9. doi: 10.1016/j.exppara.2016.04.015. Epub 2016 Apr 21. PubMed PMID: 27109312.</br>15: Ferreira FM, Palmeira CM, Oliveira MM, Santos D, Simões AM, Rocha SM, Coimbra MA, Peixoto F. Nerolidol effects on mitochondrial and cellular energetics. Toxicol In Vitro. 2012 Mar;26(2):189-96. doi: 10.1016/j.tiv.2011.11.009. Epub 2011 Nov 25. PubMed PMID: 22138475.</br>16: Houshyani B, Assareh M, Busquets A, Ferrer A, Bouwmeester HJ, Kappers IF. Three-step pathway engineering results in more incidence rate and higher emission of nerolidol and improved attraction of Diadegma semiclausum. Metab Eng. 2013 Jan;15:88-97. doi: 10.1016/j.ymben.2012.10.002. Epub 2012 Nov 12. PubMed PMID: 23154132.</br>17: Lee K, Lee JH, Kim SI, Cho MH, Lee J. Anti-biofilm, anti-hemolysis, and anti-virulence activities of black pepper, cananga, myrrh oils, and nerolidol against Staphylococcus aureus. Appl Microbiol Biotechnol. 2014 Nov;98(22):9447-57. doi: 10.1007/s00253-014-5903-4. Epub 2014 Jul 16. PubMed PMID: 25027570.</br>18: Kaur D, Pahwa P, Goel RK. Protective Effect of Nerolidol Against Pentylenetetrazol-Induced Kindling, Oxidative Stress and Associated Behavioral Comorbidities in Mice. Neurochem Res. 2016 Nov;41(11):2859-2867. Epub 2016 Jul 14. PubMed PMID: 27418279.</br>19: Utsumi S, Nakamura T, Obata Y, Ohta N, Takayama K. Effect of Nerolidol and/or Levulinic Acid on the Thermotropic Behavior of Lipid Lamellar Structures in the Stratum Corneum. Chem Pharm Bull (Tokyo). 2016;64(12):1692-1697. PubMed PMID: 27904078.</br>20: Baer P, Rabe P, Citron CA, de Oliveira Mann CC, Kaufmann N, Groll M, Dickschat JS. Hedycaryol synthase in complex with nerolidol reveals terpene cyclase mechanism. Chembiochem. 2014 Jan 24;15(2):213-6. doi: 10.1002/cbic.201300708. Epub 2014 Jan 7. PubMed PMID: 24399794.</br></br>
|