| Catalog Number | A000999 |
| CAS Number | 1405-10-3 |
| Molecular Formula | C₂₃H₄₆N₆O₁₃·3H₂SO₄
|
| Purity | 95% |
| Storage | 3 years -20C powder |
| IUPAC Name | (2R,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-[(1R,2R,3S,4R,6S)-4,6-diamino-2-[(2S,3R,4S,5R)-4-[(2R,3R,4R,5S,6S)-3-amino-6-(aminomethyl)-4,5-dihydroxyoxan-2-yl]oxy-3-hydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy-3-hydroxycyclohexyl]oxyoxane-3,4-diol;sulfuric acid |
| InChI | 1S/C23H46N6O13.H2O4S/c24-2-7-13(32)15(34)10(28)21(37-7)40-18-6(27)1-5(26)12(31)20(18)42-23-17(36)19(9(4-30)39-23)41-22-11(29)16(35)14(33)8(3-25)38-22;1-5(2,3)4/h5-23,30-36H,1-4,24-29H2;(H2,1,2,3,4)/t5-,6+,7-,8+,9-,10-,11-,12+,13-,14-,15-,16-,17-,18-,19-,20-,21-,22?,23+;/m1./s1 |
| InChIKey | OIXVKQDWLFHVGR-GQTDVWSESA-N |
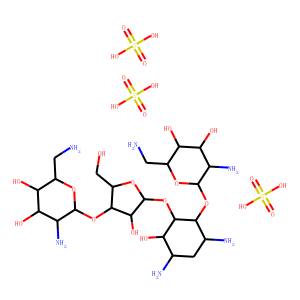
| SMILES | C1C(C(C(C(C1N)OC2C(C(C(C(O2)CN)O)O)N)OC3C(C(C(O3)CO)OC4C(C(C(C(O4)CN)O)O)N)O)O)N.OS(=O)(=O)O.OS(=O)(=O)O.OS(=O)(=O)O |
| Reference | <p>
Davis, Bernard D. /Mechanism of Bactericidal Action of Aminoglycosides./Microbiological Reviews 51.3 (1987): 341-50.</p>
<p>
Aragão F.J.L. and Brasileiro A.C.M., 2009 Positive, negative and marker-free strategies for transgenic plant selection. Braz. J. Plant Physiol., 14(1):1-10, 2002</p>
<p>
Dai S., Zheng P., Marmey P., Zhang S., Tian W., Chen S., Beachy R.N. and Fauquet C, 2001, Comparative analysis of transgenic rice plants obtained by Agrobacterium-mediated transformation and particle bombardment. Molecular Breeding 7: 25–33, 2001.</p>
<p>
Tsuji, Kiyoshi, and John H. Robertson. /Comparative Study of Responses to Neomycins B and C by Microbiological and Gas-Liquid Chromatographic Assay Methods./Applied Microbiology 18.3 (1969): 396-98. Nih.gov. Web. 23 Oct. 2013.</p>
<p>
Yuan, LingLing, and HongPing Wei. /Rapid Analysis of Native Neomycin Components on a Portable Capillary Electrophoresis System with Potential Gradient Detection./Analytical and Bioanalytical Chemistry 385.8 (2006): 1575-579. Nih.gov. Web. 23 Oct. 2013.</p>
<p>
Robertson, John H. /Antimicrobial Activity of Neomycin C Against Staphylococcus Epidermidis./ Applied Microbiology 22.6 (1971): 1164-165. Nih.gov. Web. 23 Oct. 2013.</p>
<p>
</p>
|