| Reference | 1. J Pharmacol Exp Ther. 2017 Dec;363(3):336-347. doi: 10.1124/jpet.117.242503. Epub
2017 Sep 19. <br />
Discovery and Preclinical Characterization of GSK1278863 (Daprodustat), a Small
Molecule Hypoxia Inducible Factor-Prolyl Hydroxylase Inhibitor for Anemia. <br />
Ariazi JL(1), Duffy KJ(2), Adams DF(2), Fitch DM(2), Luo L(2), Pappalardi M(2),
Biju M(2), DiFilippo EH(2), Shaw T(2), Wiggall K(2), Erickson-Miller C(2). <br />
Author information: <br />
(1)GlaxoSmithKline, King of Prussia, Pennsylvania (JLA, DFA, DF, TS, KJD, MP, MB,
KW) and while at GlaxoSmithKline, King of Prussia, Pennsylvania (LL, EHDF, CE-M)
[email protected].
(2)GlaxoSmithKline, King of Prussia, Pennsylvania (JLA, DFA, DF, TS, KJD, MP, MB,
KW) and while at GlaxoSmithKline, King of Prussia, Pennsylvania (LL, EHDF, CE-M). <br />
Decreased erythropoietin (EPO) production, shortened erythrocyte survival, and
other factors reducing the response to EPO contribute to anemia in patients who
have a variety of underlying pathologies such as chronic kidney disease.
Treatment with recombinant human EPO (rHuEPO) at supraphysiologic concentrations
has proven to be efficacious. However, it does not ameliorate the condition in
all patients, and it presents its own risks, including cardiovascular
complications. The transcription factors hypoxia-inducible factor (HIF) 1α and
HIF2α control the physiologic response to hypoxia and invoke a program of
increased erythropoiesis. Levels of HIFα are modulated by oxygen tension via the
action of a family of HIF-prolyl hydroxylases (PHDs), which tag HIFα for
proteasomal degradation. Inhibition of these PHDs simulates conditions of mild
hypoxia, leading to a potentially more physiologic erythropoietic response and
presenting a potential alternative to high doses of rHuEPO. Here we describe the
discovery and characterization of GSK1278863
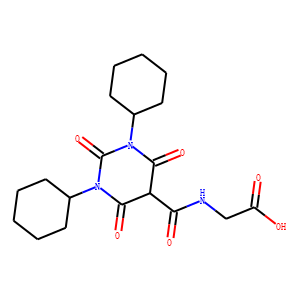
[2-(1,3-dicyclohexyl-6-hydroxy-2,4-dioxo-1,2,3,4-tetrahydropyrimidine-5-carboxami
do) acetic acid], a pyrimidinetrione-glycinamide low nanomolar inhibitor of PHDs
1-3 that stabilizes HIFα in cell lines, resulting in the production of increased
levels of EPO. In normal mice, a single dose of GSK1278863 induced significant
increases in circulating plasma EPO but only minimal increases in plasma vascular
endothelial growth factor (VEGF-A) concentrations. GSK1278863 significantly
increased reticulocytes and red cell mass parameters in preclinical species after
once-daily oral administration and has demonstrated an acceptable nonclinical
toxicity profile, supporting continued clinical development. GSK1278863 is
currently in phase 3 clinical trials for treatment of anemia in patients with
chronic kidney disease. <br />
2. Clin Pharmacol Drug Dev. 2017 Nov;6(6):627-640. doi: 10.1002/cpdd.342. Epub 2017
Apr 17. <br />
A Randomized, Placebo- and Positive-Controlled, Single-Dose, Crossover Thorough
QT/QTc Study Assessing the Effect of Daprodustat on Cardiac Repolarization in
Healthy Subjects. <br />
Caltabiano S(1), Collins J(2), Serbest G(3), Morgan L(3), Smith DA(2),
Ravindranath R(4), Cobitz AR(1). <br />
Author information: <br />
(1)Metabolic Pathways and Cardiovascular, GlaxoSmithKline, Collegeville, PA, USA.
(2)Clinical Pharmacology Modeling & Simulation, GlaxoSmithKline, Research
Triangle Park, NC, USA.
(3)Clinical Pharmacology Science & Study Operations, GlaxoSmithKline,
Collegeville, PA, USA.
(4)Clinical Statistics, GlaxoSmithKline, Bangalore, India. <br />
Daprodustat (GSK1278863) is a prolyl hydroxylase inhibitor in phase 3 clinical
studies for the treatment of anemia associated with chronic kidney disease. This
study was conducted to evaluate the effect of daprodustat on cardiac
repolarization and enrolled 55 healthy adult male (29) and female (26) subjects
who received single-dose 75 and 500 mg daprodustat, 400 mg moxifloxacin, and
placebo. Mean placebo-corrected change from baseline QT interval for daprodustat
showed no statistically significant increase. However, statistically significant
decreases in the ΔΔQTcF were observed for both doses of daprodustat, reaching a
lowest value of -2.74 milliseconds for 75 mg and -5.93 milliseconds for 500 mg
daprodustat; this minor shortening effect is not considered clinically
significant. The moxifloxacin group showed a statistically significant increase
in the ΔΔQTcF value, reaching a maximal increase of 11.47 milliseconds at
4 hours. Forty subjects (73%) reported at least 1 adverse event, with the highest
incidence with 500 mg daprodustat. This group had a higher incidence of
gastrointestinal adverse events compared to the other treatment groups. These
results suggest that 500 mg daprodustat was not well tolerated; however,
daprodustat at 75 mg was generally well tolerated. No new safety concerns were
identified in subjects who received 500 mg daprodustat. <br />
3. Am J Nephrol. 2017;45(2):127-135. doi: 10.1159/000454818. Epub 2016 Dec 16. <br />
Effects of Daprodustat, a Novel Hypoxia-Inducible Factor Prolyl Hydroxylase
Inhibitor on Anemia Management in Japanese Hemodialysis Subjects. <br />
Akizawa T(1), Tsubakihara Y, Nangaku M, Endo Y, Nakajima H, Kohno T, Imai Y,
Kawase N, Hara K, Lepore J, Cobitz A. <br />
Author information: <br />
(1)Graduate School of Health Care Sciences, Jikei Institute, Osaka, Japan. <br />
BACKGROUND: Daprodustat (GSK1278863) is an oral hypoxia-inducible factor prolyl
hydroxylase inhibitor being developed for treatment of anemia associated with
chronic kidney disease (CKD). The effect of daprodustat in Japanese CKD patients
with anemia has not been previously investigated.<br />
METHODS: We evaluated the relationship between daprodustat dose and hemoglobin
response in Japanese patients on hemodialysis (HD) with anemia in a 4-week, phase
II, double-blind, placebo-controlled study. After interrupting their
erythropoiesis-stimulating agent for between 2 and 8 weeks, subjects with
hemoglobin 8.5-10.5 g/dL were randomized to placebo or daprodustat 4, 6, 8, or 10
mg orally once daily. Hemoglobin, erythropoietin (EPO), and vascular endothelial
growth factor (VEGF) levels during therapy were evaluated.<br />
RESULTS: Eighty-six of 97 randomized subjects completed the study. Mean baseline
hemoglobin ranged from 9.68 to 9.92 g/dL across groups. After 4-week
administration, mean hemoglobin changes were -0.28, -0.01, 0.54, and 0.97 g/dL in
the 4, 6, 8, and 10 mg groups, respectively, as compared to -1.41 g/dL for
placebo. Dose-dependent increase in plasma EPO concentration were observed up to
8 mg, with the 10 mg dose responses being similar to 8 mg. Plasma VEGF
concentrations were minimally changed, even though 5 subjects treated with 6-10
mg reached EPO >500 mIU/mL.<br />
CONCLUSION: Daprodustat 4-10 mg once-daily produced dose-dependent increase in
hemoglobin relative to placebo in Japanese HD subjects. The doses evaluated in
the study have moderately increased endogenous EPO without changes in circulating
VEGF levels. <br />
|