| Catalog Number | R057001 |
| CAS Number | 514-10-3 |
| Synonyms | (1R,4aR,4bR,10aR)-1,2,3,4,4a,4b,5,6,10,10a-Decahydro-1,4a-dimethyl-7-(1-methylethyl)-1-phenanthrenecarboxylic Acid; 13-Isopropylpodocarpa-7,13-dien-15-oic Acid; (-)-Abietic Acid; 7,13-Abietadien-18-oic Acid; Abietic Acid; NSC 25149; Odomit B 10; Sylv
|
| Molecular Formula | C20H30O2
|
| Purity | 95% |
| Storage | -20°C |
| IUPAC Name | (1R,4aR,4bR,10aR)-1,4a-dimethyl-7-propan-2-yl-2,3,4,4b,5,6,10,10a-octahydrophenanthrene-1-carboxylic acid |
| InChI | InChI=1S/C20H30O2/c1-13(2)14-6-8-16-15(12-14)7-9-17-19(16,3)10-5-11-20(17,4)18(21)22/h7,12-13,16-17H,5-6,8-11H2,1-4H3,(H,21,22)/t16-,17+,19+,20+/m0/s1 |
| InChIKey | RSWGJHLUYNHPMX-ONCXSQPRSA-N |
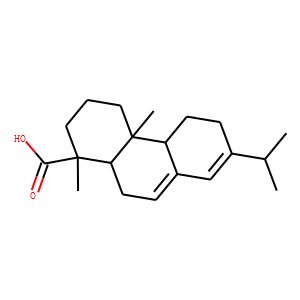
| SMILES | CC(C)C1=CC2=CCC3C(C2CC1)(CCCC3(C)C(=O)O)C |
| Reference | <span style=”font-family:arial,helvetica,sans-serif;”><span style=”font-size:12px;”><span style=”font-variant-ligatures: normal; orphans: 2; widows: 2;”>1.Liu, Xiaoqing, Wenbo Xin, and Jinwen Zhang. "Rosin-derived imide-diacids as epoxy curing agents for enhanced performance." </span><i style=”font-family: Arial, sans-serif; font-size: 13px; font-variant-ligatures: normal; orphans: 2; widows: 2;”>Bioresource technology</i><span style=”font-variant-ligatures: normal; orphans: 2; widows: 2;”> 101.7 (2010): 2520-2524.<br />
2.</span><span style=”font-variant-ligatures: normal; orphans: 2; widows: 2;”>Liu, Xiaoqing, Wenbo Xin, and Jinwen Zhang. "Rosin-based acid anhydrides as alternatives to petrochemical curing agents." </span><i style=”font-family: Arial, sans-serif; font-size: 13px; font-variant-ligatures: normal; orphans: 2; widows: 2;”>Green Chemistry</i><span style=”font-variant-ligatures: normal; orphans: 2; widows: 2;”> 11.7 (2009): 1018-1025.<br />
3.</span><span style=”font-variant-ligatures: normal; orphans: 2; widows: 2;”>Gigante, Bárbara, et al. "Structural effects on the bioactivity of dehydroabietic acid derivatives." </span><i style=”font-family: Arial, sans-serif; font-size: 13px; font-variant-ligatures: normal; orphans: 2; widows: 2;”>Planta medica</i><span style=”font-variant-ligatures: normal; orphans: 2; widows: 2;”> 68.08 (2002): 680-684.<br />
4.</span><span style=”font-variant-ligatures: normal; orphans: 2; widows: 2;”>Zapata, Bibiana, et al. "Cytotoxic, immunomodulatory, antimycotic, and antiviral activities of semisynthetic 14-hydroxyabietane derivatives and triptoquinone C-4 epimers." </span><i style=”font-family: Arial, sans-serif; font-size: 13px; font-variant-ligatures: normal; orphans: 2; widows: 2;”>MedChemComm</i><span style=”font-variant-ligatures: normal; orphans: 2; widows: 2;”> 4.9 (2013): 1239-1246.<br />
5.</span><span style=”font-variant-ligatures: normal; orphans: 2; widows: 2;”>Alvarez-Manzaneda, Enrique, et al. "Regioselective routes towards 14-hydroxyabietane diterpenes. A formal synthesis of immunosuppressant (−)-triptolide from (+)-abietic acid." </span><i style=”font-family: Arial, sans-serif; font-size: 13px; font-variant-ligatures: normal; orphans: 2; widows: 2;”>Tetrahedron</i><span style=”font-variant-ligatures: normal; orphans: 2; widows: 2;”> 63.45 (2007): 11204-11212.</span></span></span>
|