| Reference | 1. Cardiovasc Drugs Ther. 2018 Mar 26. doi: 10.1007/s10557-018-6781-2. [Epub ahead
of print]
<br>
The Caspase 1 Inhibitor VX-765 Protects the Isolated Rat Heart via the RISK
Pathway.
<br>
Do Carmo H(1), Arjun S(1)(2), Petrucci O(1), Yellon DM(3), Davidson SM(1)(2).
<br>
Author information: <br>
(1)Laboratory of Myocardial Ischemia/Reperfusion, Faculty of Medical Science,
State University of Campinas-UNICAMP, Campinas, São Paulo, Brazil.
(2)The Hatter Cardiovascular Institute, University College London, 67 Chenies
Mews, London, WC1E 6HX, UK.
(3)The Hatter Cardiovascular Institute, University College London, 67 Chenies
Mews, London, WC1E 6HX, UK. [email protected].
<br>
PURPOSE: Protecting the heart from ischaemia-reperfusion (IR) injury is a major
goal in patients presenting with an acute myocardial infarction. Pyroptosis is a
novel form of cell death in which caspase 1 is activated and cleaves interleukin
1β. VX-785 is a highly selective, prodrug caspase 1 inhibitor that is also
clinically available. It has been shown to be protective against acute IR in vivo
rat model, and therefore might be a promising possibility for future
cardioprotective therapy. However, it is not known whether protection by VX-765
involves the reperfusion injury salvage kinase (RISK) pathway. We therefore
investigated whether VX-765 protects the isolated, perfused rat heart via the
PI3K/Akt pathway and whether protection was additive with ischaemic
preconditioning (IPC).<br>
METHODS: Langendorff-perfused rat hearts were subject to ischaemia and
reperfusion injury in the presence of 30 μM VX-765, with precedent IPC, or the
combination of VX-765 and IPC.<br>
RESULTS: VX-765 reduced infarct size (28 vs 48% control; P < 0.05) to a similar
extent as IPC (30%; P < 0.05). The PI3 kinase inhibitor, wortmannin, abolished
the protective effect of VX-765. Importantly in the model used, we were unable to
show additive protection with VX-765 + IPC.<br>
CONCLUSIONS: The caspase 1 inhibitor, VX-765, was able to reduce myocardial
infarction in a model of IR injury. However, the addition of IPC did not
demonstrate any further protection.
<br>
2. J Cardiovasc Pharmacol Ther. 2017 Nov;22(6):574-578. doi:
10.1177/1074248417702890. Epub 2017 Apr 12.
<br>
The Highly Selective Caspase-1 Inhibitor VX-765 Provides Additive Protection
Against Myocardial Infarction in Rat Hearts When Combined With a Platelet
Inhibitor.
<br>
Yang XM(1), Downey JM(1), Cohen MV(1)(2), Housley NA(3)(4), Alvarez DF(1)(4),
Audia JP(3)(4).
<br>
Author information: <br>
(1)1 Department of Physiology and Cell Biology, University of South Alabama
College of Medicine, Mobile, AL, USA.
(2)2 Department of Medicine, University of South Alabama College of Medicine,
Mobile, AL, USA.
(3)3 Department of Microbiology and Immunology, University of South Alabama
College of Medicine, Mobile, AL, USA.
(4)4 Center for Lung Biology, University of South Alabama College of Medicine,
Mobile, AL, USA.
<br>
Use of ischemic postconditioning and other related cardioprotective interventions
to treat patients with acute myocardial infarction (AMI) has failed to improve
outcomes in clinical trials. Because P2Y12 inhibitors are themselves
postconditioning mimetics, it has been postulated that the loading dose of
platelet inhibitors routinely given to patients treated for AMI masks the
anti-infarct effect of other intended cardioprotective interventions. To further
improve outcomes of patients with AMI, an intervention must be able to provide
additive protection in the presence of a P2Y12 platelet inhibitor. Previous
studies reported an anti-infarct effect using a peptide inhibitor of the
pro-inflammatory caspase-1 in animal models of AMI. Herein we tested whether a
pharmacologic caspase-1 inhibitor can further limit infarct size in open-chest,
anesthetized rats treated with a P2Y12 inhibitor. One hour occlusion of a
coronary branch followed by 2 hours of reperfusion was used to simulate clinical
AMI and reflow. One group of rats received an intravenous bolus of 16 mg/kg of
the highly selective caspase-1 inhibitor VX-765 30 minutes prior to onset of
ischemia. A second group received a 60 µg/kg intravenous bolus of the P2Y12
inhibitor cangrelor 10 minutes prior to reperfusion followed by 6 µg/kg/min
continuous infusion. A third group received treatment with both inhibitors as
above. Control animals received no treatment. Infarct size was measured by
tetrazolium stain and volume of muscle at risk by fluorescent microspheres. In
untreated hearts, 73.7% ± 4.1% of the ischemic zone infarcted. Treatment with
either cangrelor or VX-765 alone reduced infarct size to 43.8% ± 2.4% and 39.6% ±
3.6% of the ischemic zone, respectively. Combining cangrelor and VX-765 was
highly protective, resulting in only 14.0% ± 2.9% infarction. The ability of
VX-765 to provide protection beyond that of a platelet inhibitor alone positions
it as an attractive candidate therapy to further improve outcomes in today’s
patients with AMI.
<br><br>
3. Org Lett. 2008 Jan 17;10(2):185-8. Epub 2007 Dec 15.
<br>
Development of a novel Pd-catalyzed N-acyl vinylogous carbamate synthesis for the
key intermediate of ICE inhibitor VX-765.
<br>
Tanoury GJ(1), Chen M, Dong Y, Forslund RE, Magdziak D.
<br>
Author information: <br>
(1)Chemical Development, Vertex Pharmaceuticals Inc., 130 Waverly Street,
Cambridge, Massachusetts 02139, USA. [email protected]
<br>
A novel Pd-catalyzed coupling of Cbz-protected proline amide with
4-bromo-5-ethoxyfuran-2(5H)-one was developed for the synthesis of the P1-P2 unit
(5) of VX-765. The process afforded quantitative coupling in the presence of
water, providing a 1:1 mixture of 5 and its ethoxy epimer epi-5. Compound 5 was
isolated as a single diastereomer via fractional crystallization, which was
stereoselectively converted to 17 via hydrogenation, and subsequently transformed
to VX-765. Nine examples of the Pd coupling are presented with yields ranging
from 76-98%.
<br><br>
4. J Pharmacol Exp Ther. 2007 May;321(2):509-16. Epub 2007 Feb 8.
<br>
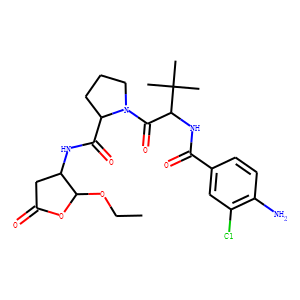
(S)-1-((S)-2-{[1-(4-amino-3-chloro-phenyl)-methanoyl]-amino}-3,3-dimethyl-butanoy
l)-pyrrolidine-2-carboxylic acid
((2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765), an orally
available selective interleukin (IL)-converting enzyme/caspase-1 inhibitor,
exhibits potent anti-inflammatory activities by inhibiting the release of
IL-1beta and IL-18.
<br>
Wannamaker W(1), Davies R, Namchuk M, Pollard J, Ford P, Ku G, Decker C,
Charifson P, Weber P, Germann UA, Kuida K, Randle JC.
<br>
Author information: <br>
(1)Department of Chemistry, Drug Discovery Support Unit, Vertex Pharmaceuticals,
Inc., 130 Waverly St., Cambridge, MA 02139, USA.
<br>
(S)-1-((S)-2-{[1-(4-amino-3-chloro-phenyl)-methanoyl]-amino}-3,3-dimethyl-butanoy
l)-pyrrolidine-2-carboxylic acid
((2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765) is an orally
absorbed prodrug of
(S)-3-({1-[(S)-1-((S)-2-{[1-(4-amino-3-chlorophenyl)-methanoyl]-amino}-3,3-dimeth
yl-butanoyl)-pyrrolidin-2yl]-methanoyl}-amino)-4-oxo-butyric acid (VRT-043198), a
potent and selective inhibitor of interleukin-converting enzyme/caspase-1
subfamily caspases. VRT-043198 exhibits 100- to 10,000-fold selectivity against
other caspase-3 and -6 to -9. The therapeutic potential of VX-765 was assessed by
determining the effects of VRT-043198 on cytokine release by monocytes in vitro
and of orally administered VX-765 in several animal models in vivo. In cultures
of peripheral blood mononuclear cells and whole blood from healthy subjects
stimulated with bacterial products, VRT-043198 inhibited the release of
interleukin (IL)-1beta and IL-18, but it had little effect on the release of
several other cytokines, including IL-1alpha, tumor necrosis factor-alpha, IL-6
and IL-8. In contrast, VRT-043198 had little or no demonstrable activity in
cellular models of apoptosis, and it did not affect the proliferation of
activated primary T cells or T-cell lines. VX-765 was efficiently converted to
VRT-043198 when administered orally to mice, and it inhibited
lipopolysaccharide-induced cytokine secretion. In addition, VX-765 reduced
disease severity and the expression of inflammatory mediators in models of
rheumatoid arthritis and skin inflammation. These data suggest that VX-765 is a
novel cytokine inhibitor useful for treatment of inflammatory diseases.
<br><br>
5. J Immunol. 2005 Aug 15;175(4):2630-4.
<br>
IL-converting enzyme/caspase-1 inhibitor VX-765 blocks the hypersensitive
response to an inflammatory stimulus in monocytes from familial cold
autoinflammatory syndrome patients.
<br>
Stack JH(1), Beaumont K, Larsen PD, Straley KS, Henkel GW, Randle JC, Hoffman HM.
<br>
Author information: <br>
(1)Vertex Pharmaceuticals, San Diego, CA 92121, USA.
<br>
Familial cold autoinflammatory syndrome (FCAS) and the related autoinflammatory
disorders, Muckle-Wells syndrome and neonatal onset multisystem inflammatory
disease, are characterized by mutations in the CIAS1 gene that encodes cryopyrin,
an adaptor protein involved in activation of IL-converting enzyme/caspase-1.
Mutations in cryopyrin are hypothesized to result in abnormal secretion of
caspase-1-dependent proinflammatory cytokines, IL-1beta and IL-18. In this study,
we examined cytokine secretion in PBMCs from FCAS patients and found a marked
hyperresponsiveness of both IL-1beta and IL-18 secretion to LPS stimulation, but
no evidence of increased basal secretion of these cytokines, or alterations in
basal or stimulated pro-IL-1beta levels. VX-765, an orally active IL-converting
enzyme/caspase-1 inhibitor, blocked IL-1beta secretion with equal potency in
LPS-stimulated cells from FCAS and control subjects. These results further link
mutations in cryopyrin with abnormal caspase-1 activation, and support the
clinical testing of caspase-1 inhibitors such as VX-765 in autoinflammatory
disorders.
<br>
|