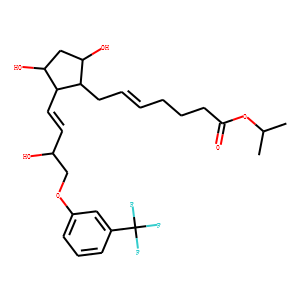
| InChI | InChI=1S/C26H35F3O6/c1-17(2)35-25(33)11-6-4-3-5-10-21-22(24(32)15-23(21)31)13-12-19(30)16-34-20-9-7-8-18(14-20)26(27,28)29/h3,5,7-9,12-14,17,19,21-24,30-32H,4,6,10-11,15-16H2,1-2H3/b5-3-,13-12+/t19-,21-,22-,23+,24-/m1/s1 |
| Reference | 1. Expert Opin Pharmacother. 2002 Jul;3(7):965-77.<br />
Travoprost–a new prostaglandin analogue for the treatment of glaucoma.<br />
Whitson JT(1).<br />
Author information:<br />
(1)Department of Ophthalmology, University of Texas, Southwestern Medical Center at Dallas, 5323 Harry Hines Boulevard, Dallas, Texas 75390-9057, USA. [email protected]<br />
Travoprost, a highly selective and potent analogue of the prostaglandin PGF(2)(alpha), has recently been approved and marketed as a topical ocular hypotensive agent for the treatment of ocular hypertension and glaucoma. Following absorption into the eye, the free acid form of travoprost interacts with the endogenous FP prostanoid receptor to enhance aqueous humor outflow and lower intraocular pressure (IOP). Travoprost is distinguished from other marketed prostaglandin analogues in that it is a full agonist at the prostaglandin receptor. It is also highly selective with little or no affinity for other prostanoid or non-prostanoid receptors in the eye. Travoprost provides robust lowering of IOP with little diurnal fluctuation and results in low target pressures in a large percentage of patients. In controlled clinical trials, travoprost 0.004% o.d. used as monotherapy produced greater IOP reduction than timolol 0.5% b.i.d. and equal or greater reduction than latanoprost 0.005%o.d. Travoprost 0.004% was also shown to be an effective adjunctive agent offering an additional 5 – 7 mmHg IOP reduction in patients inadequately controlled on timolol 0.5%. Subgroup analysis of a large Phase III trial revealed travoprost 0.004% to be significantly more effective at lowering IOP in African American patients by almost 2 mmHg compared to non-African Americans. Moreover, a higher percentage of African American patients responded to travoprost 0.004% and reached lower target pressures than with either latanoprost 0.005% or timolol 0.5%. Travoprost is a very stable compound, maintaining its efficacy following exposure to extremely low and high temperatures, repeated freezing and thawing and exposure to light. Throughout all clinical trials, travoprost was found to be safe and well-tolerated with very few (< 5%) discontinuations due to adverse events. Travoprost 0.004% represents a clinically significant advance for the treatment of glaucoma and ocular hypertension, offering superior IOP reduction and diurnal control, especially among African American patients, in a safe, well-tolerated, stable formulation.<br />
<br />
2. Surv Ophthalmol. 2002 Aug;47 Suppl 1:S105-15.<br />
Bimatoprost and travoprost: a review of recent studies of two new glaucoma drugs.<br />
Eisenberg DL(1), Toris CB, Camras CB.<br />
Author information:<br />
(1)Shepherd Eye Center, Las Vegas, NV, USA.<br />
Bimatoprost (Lumigan [Allergan, Inc, Irvine CA]) and travoprost (Travatan [Alcon, Ft Worth, TX]) are two new intraocular pressure (IOP)-lowering drugs for use in patients with glaucoma and ocular hypertension. This review evaluates recent studies comparing these new drugs with timolol and with latanoprost. In each study, the statistical analyses support the conclusion that these agents were more effective than timolol and as effective as latanoprost in terms of their ability to reduce IOP. The side effect profiles for bimatoprost, latanoprost, and travoprost were similar, but with statistically higher occurrences of hyperemia and eyelash growth for bimatoprost or travoprost versus latanoprost or timolol.<br />
<br />
3. Drugs Today (Barc). 2003 Jan;39(1):61-74.<br />
Travoprost: a potent ocular hypotensive agent.<br />
Al-Jazzaf AM(1), DeSantis L, Netland PA.<br />
Author information:<br />
(1)Department of Ophthalmology, University of Tennessee Health Science Center, 956 Court Avenue, Suite D228, Memphis, TN 38163, USA.<br />
Travoprost (isopropyl (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(1E,3R)-3-hydroxy-4-[alpha,alpha,alpha,trif luoro-m-tolyl)oxy]-1butenyl]cyclopentyl]-5-heptenoate) is an isopropyl ester prodrug and a high-affinity, selective FP prostaglandin- receptor full agonist. This prodrug is a synthetic prostaglandin analogue, which in appropriate cases is administered topically for the treatment of glaucoma and ocular hypertension. The isopropyl ester prodrug is rapidly hydrolyzed by esterases in the cornea to the biologically active, free acid. Travoprost has demonstrated preferential affinity and full agonist activity for the FP receptor in the nanomolar range, with no meaningful affinity or activity at other receptors. Like other compounds of this class, the reduction of intraocular pressure by travoprost is due at least in part to increased uveoscleral outflow. Results from phase II and phase III pivotal studies for FDA approval in the United States have demonstrated that travoprost is an effective topical agent for treatment of elevated intraocular pressure in patients with open-angle glaucoma and ocular hypertension. Travoprost is a safe drug, with local side effects including hyperemia, eyelash growth and iris color change. The dosing is once per day in the evening, and storage does not require refrigeration. Travoprost will be a helpful new drug in the medical management of glaucoma.<br />
|