| Reference | 1. Diabetes Obes Metab. 2012 Mar;14(3):271-8. doi: 10.1111/j.1463-1326.2011.01525.x.
Epub 2011 Dec 22.
<br>
GPR40-induced insulin secretion by the novel agonist TAK-875: first clinical
findings in patients with type 2 diabetes.
<br>
Araki T(1), Hirayama M, Hiroi S, Kaku K.
<br>
Author information: <br>
(1)Pharmaceutical Development Division, Takeda Pharmaceutical Company Limited,
Osaka, Japan. [email protected]
<br>
AIM: Free fatty acids act as signalling molecules for modulating insulin
secretion, and their insulinotropic effects are glucose-dependent and mediated
through G protein-coupled receptor 40 (GPR40). This mechanism is a potential
target for new treatments for managing diabetes. In this study, we present the
first clinical data for TAK-875, a novel highly selective, orally bioavailable
GPR40 agonist, in Japanese patients with type 2 diabetes insufficiently
controlled by diet or exercise therapy.<br>
METHODS: This was an exploratory phase II, multicentre, randomized, double-blind,
parallel group study comparing the efficacy and tolerability of TAK-875 100 and
400 mg, and placebo, all administered once daily for 2 weeks.
RESULTS: After 2 weeks of treatment, TAK-875 produced marked glucose lowering
effects in a 75 g oral glucose tolerance test (OGTT) as evidenced by mean ± SE
intergroup differences in plasma glucose AUC(0-3 h) of -12.98 ± 1.48 (p < 0.0001)
and -8.12 ± 1.49 mmol·h/l (p < 0.0001), for TAK-875 400 mg vs. placebo and
TAK-875 100 mg vs. placebo, respectively, and 2 h plasma glucose [-4.95 ± 0.71 (p
< 0.0001) and -3.21 ± 0.71 mmol/l (p < 0.0001), respectively]. This was
accompanied by a significant increase in insulin AUC(0-3 h) [34.68 ± 12.16 (p <
0.01) and 31.49 ± 12.20 (p < 0 · 05) μIU·h/ml, respectively]. Improvement in
glycaemic profile was mirrored by a significant change in fasting plasma glucose
[-2.37 ± 0·27 (p < 0.0001) and -1.88 ± 0.27 mmol/l (p < 0.0001), respectively].
No cases of hypoglycaemia were observed despite the significant reduction in
plasma glucose.<br>
CONCLUSIONS: These exploratory findings provide evidence of the glucose-dependent
insulinotropic potential of the GPR40 agonist TAK-875, and the promising clinical
changes support future longer term clinical investigation.
<br>
2. ACS Med Chem Lett. 2010 Jun 18;1(6):290-4. doi: 10.1021/ml1000855. eCollection
2010 Sep 9.
<br>
Discovery of TAK-875: A Potent, Selective, and Orally Bioavailable GPR40 Agonist.
<br>
Negoro N(1), Sasaki S(1), Mikami S(1), Ito M(1), Suzuki M(1), Tsujihata Y(1), Ito
R(1), Harada A(1), Takeuchi K(1), Suzuki N(1), Miyazaki J(1), Santou T(1), Odani
T(1), Kanzaki N(1), Funami M(1), Tanaka T(1), Kogame A(1), Matsunaga S(1), Yasuma
T(1), Momose Y(1).
<br>
Author information: <br>
(1)Pharmaceutical Research Division, Takeda Pharmaceutical Company, Ltd.,
2-17-85, Jusohonmachi, Yodogawa-ku, Osaka 532-8686, Japan.
<br>
GPR40, one of the G protein-coupled receptors predominantly expressed in
pancreatic β-cells, mediates enhancement of glucose-stimulated insulin secretion
by free fatty acids. A potent and selective GPR40 agonist is theorized to be a
safe and effective antidiabetic drug with little or no risk of hypoglycemia.
Cyclization of the phenylpropanoic acid moiety of lead compound 1 produced fused
phenylalkanoic acids with favorable in vitro agonist activities and
pharmacokinetic profiles. Further optimization led to the discovery of
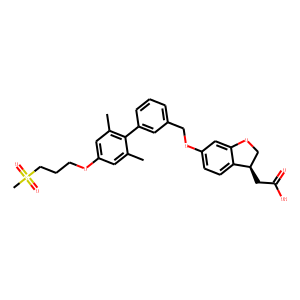
dihydrobenzofuran derivative 9a
([(3S)-6-({2/’,6/’-dimethyl-4/’-[3-(methylsulfonyl)propoxy]biphenyl-3-yl}methoxy)-2,
3-dihydro-1-benzofuran-3-yl]acetic acid hemi-hydrate, TAK-875) as a potent,
selective, and orally bioavailable GPR40 agonist, with a pharmacokinetic profile
enabling long-acting drug efficacy. Compound 9a showed potent plasma
glucose-lowering action and insulinotropic action during an oral glucose
tolerance test in female Wistar fatty rats with impaired glucose tolerance.
Compound 9a is currently in clinical trials for the treatment of type 2 diabetes
mellitus.
<br>
|