| Reference | 1. Antimicrob Agents Chemother. 2017 Dec 21;62(1). pii: e01620-17. doi: 10.1128/AAC.01620-17. Print 2018 Jan.<br />
In Vitro Antiviral Activity and Resistance Profile of the Next-Generation Hepatitis C Virus NS3/4A Protease Inhibitor Glecaprevir.<br />
Ng TI(1), Tripathi R(2), Reisch T(2), Lu L(2), Middleton T(2), Hopkins TA(2), Pithawalla R(2), Irvin M(2), Dekhtyar T(2), Krishnan P(2), Schnell G(2), Beyer J(2), McDaniel KF(2), Ma J(3), Wang G(3), Jiang LJ(3), Or YS(3), Kempf D(2), Pilot-Matias T(2), Collins C(2).<br />
Author information:<br />
(1)AbbVie, Inc., North Chicago, Illinois, USA [email protected]. (2)AbbVie, Inc., North Chicago, Illinois, USA. (3)Enanta Pharmaceuticals Inc., Watertown, Massachusetts, USA.<br />
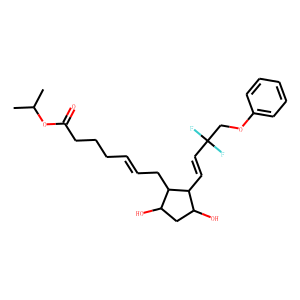
Glecaprevir (formerly ABT-493) is a novel hepatitis C virus (HCV) NS3/4A protease inhibitor (PI) with pangenotypic activity. It inhibited the enzymatic activity of purified NS3/4A proteases from HCV genotypes 1 to 6 in vitro (half-maximal [50%] inhibitory concentration = 3.5 to 11.3 nM) and the replication of stable HCV subgenomic replicons containing proteases from genotypes 1 to 6 (50% effective concentration [EC50] = 0.21 to 4.6 nM). Glecaprevir had a median EC50 of 0.30 nM (range, 0.05 to 3.8 nM) for HCV replicons containing proteases from 40 samples from patients infected with HCV genotypes 1 to 5. Importantly, glecaprevir was active against the protease from genotype 3, the most-difficult-to-treat HCV genotype, in both enzymatic and replicon assays demonstrating comparable activity against the other HCV genotypes. In drug-resistant colony selection studies, glecaprevir generally selected substitutions at NS3 amino acid position A156 in replicons containing proteases from genotypes 1a, 1b, 2a, 2b, 3a, and 4a and substitutions at position D/Q168 in replicons containing proteases from genotypes 3a, 5a, and 6a. Although the substitutions A156T and A156V in NS3 of genotype 1 reduced susceptibility to glecaprevir, replicons with these substitutions demonstrated a low replication efficiency in vitro Glecaprevir is active against HCV with most of the common NS3 amino acid substitutions that are associated with reduced susceptibility to other currently approved HCV PIs, including those at positions 155 and 168. Combination of glecaprevir with HCV inhibitors with other mechanisms of action resulted in additive or synergistic antiviral activity. In summary, glecaprevir is a next-generation HCV PI with potent pangenotypic activity and a high barrier to the development of resistance.<br />
2. Lancet Infect Dis. 2017 Oct;17(10):1062-1068. doi: 10.1016/S1473-3099(17)30496-6. Epub 2017 Aug 14.<br />
Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial.<br />
Forns X(1), Lee SS(2), Valdes J(3), Lens S(4), Ghalib R(5), Aguilar H(6), Felizarta F(7), Hassanein T(8), Hinrichsen H(9), Rincon D(10), Morillas R(11), Zeuzem S(12), Horsmans Y(13), Nelson DR(14), Yu Y(3), Krishnan P(3), Lin CW(3), Kort JJ(3), Mensa FJ(3).<br />
BACKGROUND AND OBJECTIVE: Glecaprevir and pibrentasvir are pangenotypic direct-acting antiviral agents for the treatment of chronic hepatitis C virus infection. The aim of the present study was to evaluate the drug-drug interaction and safety of glecaprevir and pibrentasvir coadministration in healthy volunteers.<br />
METHODS: In this open-label, randomized, multiple-dose, Phase 1 study in 72 subjects, glecaprevir (100-1200 mg once daily) and pibrentasvir (40-200 mg once daily) were administered alone for 7 days and then in combination for another 7 days. Intensive blood sampling was performed on Days 1, 7, 8, and 14, and pharmacokinetic interactions were assessed using a repeated measures analysis of glecaprevir and pibrentasvir maximum plasma concentration (C max) and area under the curve (AUC).<br />
RESULTS: Coadministration of glecaprevir 400 mg increased pibrentasvir 120 and 40 mg steady-state C max and AUC values to 2.9-6.3-fold, and coadministration of glecaprevir 700 mg increased pibrentasvir 160 mg steady-state C max and AUC24 values to up to sevenfold of the values when pibrentasvir was administered alone. Glecaprevir C max and AUC values during coadministration were less than 1.5-fold of the values when glecaprevir was administered alone. The combination of glecaprevir and pibrentasvir at doses up to 400 mg was well tolerated by the healthy subjects in this study. High glecaprevir exposures at 700 and 1200 mg were associated with grade 2/3 elevations in alanine aminotransferase, aspartate aminotransferase, and/or bilirubin.<br />
CONCLUSIONS: Coadministration of pibrentasvir 120 mg with glecaprevir doses up to 400 mg resulted in increases in pibrentasvir exposures without significant changes in glecaprevir exposures in the absence of any clinically significant laboratory abnormalities.<br />
|