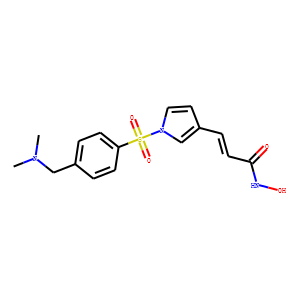
| Reference | 1: Enzenhofer E, Kadletz L, Stanisz I, Kotowski U, Seemann R, Schmid R, Thurnher
D, Heiduschka G. Effect of the histone deacetylase inhibitor resminostat on head
and neck squamous cell carcinoma cell lines. Head Neck. 2017 May;39(5):900-907.
doi: 10.1002/hed.24699. Epub 2017 Feb 7. PubMed PMID: 28170128.
<br>
2: Tambo Y, Hosomi Y, Sakai H, Nogami N, Atagi S, Sasaki Y, Kato T, Takahashi T,
Seto T, Maemondo M, Nokihara H, Koyama R, Nakagawa K, Kawaguchi T, Okamura Y,
Nakamura O, Nishio M, Tamura T. Phase I/II study of docetaxel combined with
resminostat, an oral hydroxamic acid HDAC inhibitor, for advanced non-small cell
lung cancer in patients previously treated with platinum-based chemotherapy.
Invest New Drugs. 2017 Apr;35(2):217-226. doi: 10.1007/s10637-017-0435-2. Epub
2017 Jan 30. PubMed PMID: 28138828.
<br>
3: Ellerhoff TP, Berchtold S, Venturelli S, Burkard M, Smirnow I, Wulff T, Lauer
UM. Novel epi-virotherapeutic treatment of pancreatic cancer combining the oral
histone deacetylase inhibitor resminostat with oncolytic measles vaccine virus.
Int J Oncol. 2016 Nov;49(5):1931-1944. doi: 10.3892/ijo.2016.3675. Epub 2016 Aug
31. PubMed PMID: 27601235.
<br>
4: Peng X, Zhang D, Li Z, Fu M, Liu H. mTOR inhibition sensitizes human
hepatocellular carcinoma cells to resminostat. Biochem Biophys Res Commun. 2016
Sep 2;477(4):556-562. doi: 10.1016/j.bbrc.2016.06.060. Epub 2016 Jun 14. PubMed
PMID: 27311860.
<br>
5: Fu M, Shi W, Li Z, Liu H. Activation of mPTP-dependent mitochondrial apoptosis
pathway by a novel pan HDAC inhibitor resminostat in hepatocellular carcinoma
cells. Biochem Biophys Res Commun. 2016 Sep 2;477(4):527-533. doi:
10.1016/j.bbrc.2016.04.147. Epub 2016 May 1. PubMed PMID: 27144317.
<br>
6: Bitzer M, Horger M, Giannini EG, Ganten TM, Wörns MA, Siveke JT, Dollinger MM,
Gerken G, Scheulen ME, Wege H, Zagonel V, Cillo U, Trevisani F, Santoro A,
Montesarchio V, Malek NP, Holzapfel J, Herz T, Ammendola AS, Pegoraro S, Hauns B,
Mais A, Lauer UM, Henning SW, Hentsch B. Resminostat plus sorafenib as
second-line therapy of advanced hepatocellular carcinoma – The SHELTER study. J
Hepatol. 2016 Aug;65(2):280-8. doi: 10.1016/j.jhep.2016.02.043. Epub 2016 Mar 4.
PubMed PMID: 26952006.
<br>
7: Zhao J, Lawless MW. Resminostat: Opening the door to epigenetic treatments for
liver cancer. Hepatology. 2016 Feb;63(2):668-9. doi: 10.1002/hep.27853. Epub 2015
May 29. PubMed PMID: 25891162.
<br>
8: Kitazono S, Fujiwara Y, Nakamichi S, Mizugaki H, Nokihara H, Yamamoto N,
Yamada Y, Inukai E, Nakamura O, Tamura T. A phase I study of resminostat in
Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2015
Jun;75(6):1155-61. doi: 10.1007/s00280-015-2741-8. Epub 2015 Apr 7. PubMed PMID:
25847480.
<br>
9: Ruf B, Berchtold S, Venturelli S, Burkard M, Smirnow I, Prenzel T, Henning SW,
Lauer UM. Combination of the oral histone deacetylase inhibitor resminostat with
oncolytic measles vaccine virus as a new option for epi-virotherapeutic treatment
of hepatocellular carcinoma. Mol Ther Oncolytics. 2015 Oct 7;2:15019. doi:
10.1038/mto.2015.19. eCollection 2015. PubMed PMID: 27119111; PubMed Central
PMCID: PMC4782956.
<br>
10: Brunetto AT, Ang JE, Lal R, Olmos D, Molife LR, Kristeleit R, Parker A,
Casamayor I, Olaleye M, Mais A, Hauns B, Strobel V, Hentsch B, de Bono JS.
First-in-human, pharmacokinetic and pharmacodynamic phase I study of Resminostat,
an oral histone deacetylase inhibitor, in patients with advanced solid tumors.
Clin Cancer Res. 2013 Oct 1;19(19):5494-504. doi: 10.1158/1078-0432.CCR-13-0735.
Epub 2013 Sep 24. PubMed PMID: 24065624; PubMed Central PMCID: PMC3790647.
|