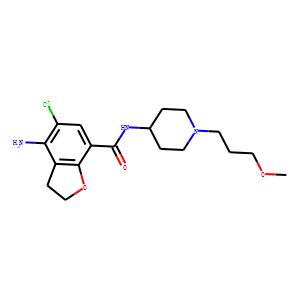
| Reference | [1]. Prucalopride.<br />
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. 2019 Apr 25.<br />
Prucalopride is a serotonin type 4 (5-HT4) receptor agonist that has potent prokinetic activity and is used as therapy for chronic idiopathic constipation. Prucalopride has been associated with a minimal rate of transient serum enzyme elevations during therapy and has not been implicated in cases of clinically apparent liver injury with jaundice.<br />
PMID: 31643961<br />
<br />
[2]. Am J Gastroenterol. 2019 Aug;114(8):1265-1274. doi: 10.14309/ajg.0000000000000304.<br />
Prucalopride in Gastroparesis: A Randomized Placebo-Controlled Crossover Study.<br />
Carbone F(1), Van den Houte K(1), Clevers E(1), Andrews CN(1), Papathanasopoulos A(1), Holvoet L(1), Van Oudenhove L(1), Caenepeel P(1), Arts J(1), Vanuytsel T(1), Tack J(1).<br />
Author information: (1)Translational Research Center for Gastrointestinal Disorders (TARGID), University of Leuven, Leuven, Belgium.<br />
Comment in Am J Gastroenterol. 2019 Dec;114(12):1919. Am J Gastroenterol. 2019 Dec;114(12):1920-1921.<br />
OBJECTIVES: Prokinetics are considered the preferred treatment option for gastroparesis, but evidence of their efficacy is scarce. Prucalopride, a selective 5-hydroxytryptamine 4 receptor agonist used in the treatment of constipation, is able to enhance the gastric emptying rate. In a double-blind, randomized, placebo-controlled crossover study, we evaluated the efficacy of prucalopride to improve the gastric emptying rate and symptoms in patients with gastroparesis. METHODS: Thirty-four patients with gastroparesis (28 idiopathic, 7 men, mean age 42 ± 13 years) were evaluated in a double-blind crossover trial of 4-week treatment periods with placebo or prucalopride 2 mg q.d., separated by 2 weeks of washout. The primary end point was the change in symptom severity, assessed by the Gastroparesis Cardinal Symptom Index; secondary end points comprised the Patient Assessment of Upper Gastrointestinal Disorders-Symptom Severity Index, the Patient Assessment of Upper Gastrointestinal Disorders-Quality of Life, and daily diaries, and the gastric emptying rate was assessed by the C-octanoic acid breath test. RESULTS: Three patients were lost to follow-up. One serious adverse event occurred (small bowel volvulus in the prucalopride group), and 3 patients dropped out because of adverse events of nausea and headache (all prucalopride). For the entire patient group, compared with placebo, prucalopride significantly improved the total Gastroparesis Cardinal Symptom Index (1.65 ± 0.19 vs 2.28 ± 0.20, P < 0.0001) and the subscales of fullness/satiety, nausea/vomiting, and bloating/distention. Prucalopride significantly improved the overall Patient Assessment of Upper Gastrointestinal Disorders-Quality of Life score (1.15 ± 0.16 vs 1.44 ± 0.16, P < 0.05) and the domains of clothing and diet. The gastric half emptying time was significantly enhanced by prucalopride compared with placebo and baseline (98 ± 10 vs 143 ± 11 and 126 ± 13 minutes, P = 0.005 and <0.001, respectively). These significant improvements were also found when considering only the idiopathic gastroparesis subgroup. DISCUSSION: In a cohort of patients with predominantly idiopathic gastroparesis, 4 weeks of prucalopride treatment significantly improved symptoms and quality of life and enhanced gastric emptying compared with placebo.<br />
DOI: 10.14309/ajg.0000000000000304 PMID: 31295161 [Indexed for MEDLINE]<br />
<br />
[3]. Expert Rev Clin Pharmacol. 2019 Jul;12(7):579-589. doi: 10.1080/17512433.2019.1620104. Epub 2019 May 23.<br />
Use of prucalopride in adults with chronic idiopathic constipation.<br />
Vijayvargiya P(1), Camilleri M(1).<br />
Author information: (1)a Clinical Enteric Neuroscience Translational and Epidemiological Research (C.E.N.T.E.R.), Division of Gastroenterology and Hepatology , Mayo Clinic , Rochester , MN , USA.<br />
Introduction: Prucalopride is a selective 5-HT4 receptor agonist with colonic prokinetic activity. It was recently approved by the FDA for the treatment of chronic idiopathic constipation. Before this approval, there were limited options to improve colonic motility in the treatment of chronic idiopathic constipation. Areas covered: We systematically searched PubMed, Embase, ClinicalTrials.gov, and international conference presentations, and we reviewed all studies that evaluated prucalopride for the treatment of chronic idiopathic constipation in adults. In this review, we discuss the pharmacokinetics, pharmacodynamics, receptor interactions, phase I-IV clinical trials, and safety outcomes of prucalopride in adults, including the elderly. Expert opinion: Prucalopride is an effective agent to improve colonic motility, decrease colonic transit time, and increase complete spontaneous bowel movements in patients with chronic idiopathic constipation. Unlike previously available 5-HT4 receptor agonists such as cisapride and tegaserod, prucalopride does not interact with the cardiac hERG potassium channels or other serotonergic receptors in blood vessels and is not associated with an increase in major adverse cardiovascular events. Additionally, prucalopride has demonstrated promise in the treatment of gastroparesis, post-operative ileus, and opioid-induced constipation. Prucalopride directly stimulates colonic motility, differentiating it from all other medications (exclusively osmotic or chloride secretagogues) approved for chronic constipation in the last decade.<br />
DOI: 10.1080/17512433.2019.1620104 PMID: 31096799 [Indexed for MEDLINE]<br />
<br />
[4]. Expert Rev Gastroenterol Hepatol. 2019 Mar;13(3):257-262. doi: 10.1080/17474124.2019.1568238. Epub 2019 Jan 23.<br />
Prucalopride for the treatment of constipation: a view from 2015 and beyond.<br />
Bassotti G(1), Usai Satta P(2), Bellini M(3).<br />
Author information: (1)a Gastroenterology & Hepatology Section, Department of Medicine , University of Perugia Medical School , Perugia , Italy. (2)b Gastrointestinal Unit , "G. Brotzu" Hospital , Cagliari , Italy. (3)c Gastrointestinal Unit, Department of Translational Research and New Technologies in Medicine and Surgery , University of Pisa , Pisa , Italy.<br />
Prucalopride is a prokinetic drug, that has been commercially available in recent years for the treatment of chronically constipated patients. In this update of a previous 2016 article, we reviewed the more recent data supporting its role in the treatment of constipation and constipation-associated conditions. Areas covered: We carried out an extensive literature review on the effects of prucalopride for the years 2012-2018 by means of scientific databases and manual research. More evidence was found on its possible therapeutic role in conditions in which constipation plays a role as an associated symptom, such as opioid-induced constipation, constipation-predominant irritable bowel syndrome, post-operative ileus, colonic diverticular disease, drug-related constipation, and chronic intestinal pseudo-obstruction. Expert opinion: Based on the added literature evidence, we feel that prucalopride is an effective, although expensive, drug for the treatment of primary and secondary forms of constipation, and of other clinical conditions associated with constipation.<br />
DOI: 10.1080/17474124.2019.1568238 PMID: 30791758 [Indexed for MEDLINE]<br />
<br />
[5]. Expert Opin Pharmacother. 2010 Feb;11(3):451-61. doi: 10.1517/14656560903567057.<br />
Prucalopride for constipation.<br />
Camilleri M(1), Deiteren A.<br />
Author information: (1)Clinical Enteric Neuroscience Translational and Epidemiological Research (C.E.N.T.E.R.), College of Medicine, Mayo Clinic, Charlton 8-110, 200 First St S.W., Rochester, MN 55905, USA. [email protected]<br />
IMPORTANCE OF THE FIELD: Chronic constipation has a high prevalence, and current medical and pharmacological therapies do not restore normal bowel function in all patients. AREAS COVERED IN THE REVIEW: A PubMed search (1965 – 2009) using the following terms alone or in combination: prucalopride, 5-HT(4), R093877, safety, toxicity, pharmacokinetics, pharmacodynamics, transit, cardiac, hERG, arrhythmia, potassium current, elderly. WHAT THE READER WILL GAIN: Understanding of the mechanisms of action, safety, efficacy and indications for prucalopride in patients with chronic constipation. TAKE HOME MESSAGE: Prucalopride is an efficacious and generally safe, new therapeutic option in the management of chronic constipation.<br />
DOI: 10.1517/14656560903567057 PMID: 20102308 [Indexed for MEDLINE]
|