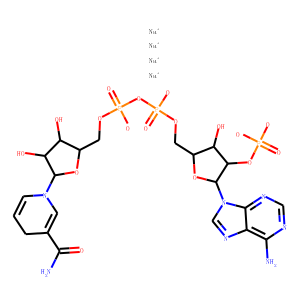
| Reference | [1]. Talanta. 2019 Jul 1;199:573-580. doi: 10.1016/j.talanta.2019.03.018. Epub 2019 Mar 2.<br />
Novel molecularly imprinted polymer (MIP) multiple sensors for endogenous redox couples determination and their applications in lung cancer diagnosis.<br />
Liu J(1), Wang Y(2), Liu X(3), Yuan Q(4), Zhang Y(5), Li Y(6).<br />
Author information: (1)College of Science, Harbin Institute of Technology, Shenzhen 518055, China; Key Laboratory of Xinjiang Phytomedicine Resource and Utilization, Ministry of Education, School of Pharmacy, Shihezi University, Shihezi 832000, China. (2)The first affiliated hospital of the medical college of Shihezi University, Shihezi 832000, China. (3)College of Science, Harbin Institute of Technology, Shenzhen 518055, China. (4)College of Materials Science and Engineering, Harbin Institute of Technology, Shenzhen 518055, China. (5)College of Science, Harbin Institute of Technology, Shenzhen 518055, China. Electronic address: [email protected]. (6)College of Science, Harbin Institute of Technology, Shenzhen 518055, China; Key Laboratory of Xinjiang Phytomedicine Resource and Utilization, Ministry of Education, School of Pharmacy, Shihezi University, Shihezi 832000, China. Electronic address: [email protected].<br />
Multiplex electrochemical sensors for amperometric detection of glutathione disulfide (GSSG), glutathione (GSH), cysteine (Cys), cystine (Cyss), β-nicotinamide adenine dinucleotide phosphate (NADP+) and coenzyme II reduced tetrasodium salt (NADPH) were developed, in which analysis of Cyss, NADP+ and NADPH are the first report using this sensing system. Specificity of these sensors were ensured by a layer of molecularly imprinted polymer (MIP) which was electropolymerized in situ with the analytes as template. All the sensors were tested with standard buffers and mouse blood samples, showing satisfactory performance towards the corresponding analytes. Dynamic concentration for the six analytes was in the range of 10-11-10-8 mol/L with the detection limit down to 20 pmol/L. In addition, artificially synthesized MIP film on the electrodes allowed for good selectivity and stability. Real blood sample measurement proved that the sensors owned decent accuracy with recovery value ranging from 92%~112%. More importantly, blood samples from lung cancer patients and healthy donors were assayed by using the proposed sensors. Redox potentials (Ehc) were calculated based on the contents of these endogenic substances, which were utilized to reflect the health status of human body and help diagnose lung cancer. The levels of GSH, NADPH and the absolute value of Ehc(GSH/GSSG) in patients with lung cancer are significantly lower (P < 0.01) than those in healthy people, while the contents of GSSG (P < 0.01) are higher. The blood test results suggested that the content of GSH, NADPH, NADP+ and Ehc(GSH/GSSG) might serve as biomarkers for lung cancer prediagnosis. These novel sensors for liquid biospy of cancer have cost-benefit and scalability advantage over current techniques, potentially enabling broader clinical access and efficient population screening.<br />
DOI: 10.1016/j.talanta.2019.03.018 PMID: 30952300<br />
<br />
[2]. Insect Mol Biol. 2017 Oct;26(5):543-551. doi: 10.1111/imb.12317. Epub 2017 Jun 27.<br />
Imidacloprid is hydroxylated by Laodelphax striatellus CYP6AY3v2.<br />
Wang R(1), Zhu Y(1), Deng L(1), Zhang H(1), Wang Q(1), Yin M(2), Song P(2), Elzaki MEA(1), Han Z(1), Wu M(1).<br />
Author information: (1)Department of Entomology, College of Plant Protection, Nanjing Agricultural University, Jiangsu/Key Laboratory of Monitoring and Management of Plant Diseases and Insects, Ministry of Agriculture, Nanjing, Jiangsu, China. (2)Jiangsu Center for Research & Development of Medicinal Plants, Institute of Botany, Jiangsu Province and the Chinese Academy of Sciences, Nanjing, Jiangsu, China.<br />
Laodelphax striatellus (Fallén) is one of the most destructive pests of rice, and has developed high resistance to imidacloprid. Our previous work indicated a strong association between imidacloprid resistance and the overexpression of a cytochrome P450 gene CYP6AY3v2 in a L. striatellus imidacloprid resistant strain (Imid-R). In this study, a transgenic Drosophila melanogaster line that overexpressed the L. striatellus CYP6AY3v2 gene was established and was found to confer increased levels of imidacloprid resistance. Furthermore, CYP6AY3v2 was co-expressed with D. melanogaster cytochrome P450 reductase (CPR) in Spodoptera frugiperda 9 (SF9) cells. A carbon monoxide difference spectra analysis indicated that CYP6AY3v2 was expressed predominately in its cytochrome P450 (P450) form, which is indicative of a good-quality functional enzyme. The recombinant CYP6AY3v2 protein efficiently catalysed the model substrate P-nitroanisole to p-nitrophenol with a maximum velocity (Vmax ) of 60.78 ± 3.93 optical density (mOD)/min/mg protein. In addition, imidacloprid itself was metabolized by the recombinant CYP6AY3v2/nicotinamide adenine dinucleotide 2'-phosphate reduced tetrasodium salt (NADPH) CPR microsomes in in vitro assays (catalytic constant (Kcat ) = 0.34 pmol/min/pmol P450, michaelis constant (Km ) = 41.98 μM), and imidacloprid depletion and metabolite peak formation were with a time dependence. The data provided direct evidence that CYP6AY3v2 is capable of hydroxylation of imidacloprid and conferring metabolic resistance in L. striatellus.<br />
DOI: 10.1111/imb.12317 PMID: 28654199<br />
<br />
[3]. Biomed Chromatogr. 2020 Apr;34(4):e4806. doi: 10.1002/bmc.4806. Epub 2020 Feb 13.<br />
Characterization of the metabolites of rosmarinic acid in human liver microsomes using liquid chromatography combined with electrospray ionization tandem mass spectrometry.<br />
Su J(1), Jia F(1), Lu J(2), Chen W(1), Sun H(1), Liu T(1), Wu X(1).<br />
Author information: (1)Department of Gastroenterology, Xuzhou Central Hospital, The Xuzhou School of Clinical Medicine of Nanjing Medical University, Xuzhou, Jiangsu Province, China. (2)Department of Neurology, Xuzhou Central Hospital, The Xuzhou School of Clinical Medicine of Nanjing Medical University, Xuzhou, Jiangsu Province, China.<br />
Rosmarinic acid (RA) is a phenolic acid originally isolated from the herb medicine Rosmarinus officinalis. The purpose of this study was to identify the metabolites of RA. RA was incubated with human liver microsomes in the presence of β-nicotinamide adenine dinucleotide phosphate tetrasodium salt and/or uridine diphosphate glucuronic acid using glutathione (GSH) as a trapping agent. After 60-min incubation, the samples were analyzed using high-resolution liquid chromatography tandem mass spectrometry. Under the current conditions, 14 metabolites were detected and identified. Our data revealed that RA was metabolized through the following pathways: the first pathway is the oxidation of catechol to form ortho-quinone intermediates, which react with GSH to form mono-GSH adducts (M1, M2, and M3) and bis-GSH adducts (M4 and M5); the second pathway is conjugation with glucuronide to yield acylglucuronide (M7), which further reacts with GSH to form RA-S-acyl-GSH adduct (M9); the third pathway is hydroxylation to form M10, M11, and M12, which further react with GSH to form mono-GSH adducts (M13 and M14); the fourth pathway is conjugation with GSH through Michael addition (M6); the fifth pathway is conjugation with glucuronidation, forming M8, which is the major metabolic pathway of RA.<br />
DOI: 10.1002/bmc.4806 PMID: 32012312<br />
<br />
[4]. Anal Chim Acta. 2013 Jul 5;786:111-5. doi: 10.1016/j.aca.2013.04.067. Epub 2013 May 13.<br />
Fluorescence detection of glutathione reductase activity based on deoxyribonucleic acid-templated silver nanoclusters.<br />
Zhu S(1), Zhao XE, Zhang W, Liu Z, Qi W, Anjum S, Xu G.<br />
Author information: (1)State Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, Jilin, China.<br />
Fluorescent silver nanoclusters stabilized by DNA (DNA-AgNCs) exhibit distinct response rates to thiol and disulfide. Glutathione reductase can catalyze the reduction of the oxidized glutathione (GSSG) quickly to reduced glutathione (GSH) in the presence of β-nicotinamide adenine dinucleotide 2'-phosphate reduced tetrasodium salt hydrate (NADPH). Consequently, DNA-AgNCs can serve as a new fluorescent platform for assaying the glutathione reductase (GR) activity. This newly proposed assay has a high sensitivity and a good selectivity toward GR. The GR activity can be detected in the range of 0.2-2.0 mU mL(-1) with a minimum detectable concentration of 0.2 mU mL(-1). Pepsin, lysozyme, trypsin, avidin, thrombin, myoglobin, and BSA have little effect on the fluorescence intensity of DNA-AgNCs. The GR activity assay is successfully used to monitor the inhibition of GR activity by a typical inhibitor 1,3-bis(2-chloroethyl)-1-nitrosourea.<br />
DOI: 10.1016/j.aca.2013.04.067 PMID: 23790299<br />
<br />
[5]. Nanoscale Res Lett. 2014 Nov 28;9(1):642. doi: 10.1186/1556-276X-9-642. eCollection 2014.<br />
Size- and time-dependent alteration in metabolic activities of human hepatic cytochrome P450 isozymes by gold nanoparticles via microsomal coincubations.<br />
Ye M(1), Tang L(1), Luo M(2), Zhou J(1), Guo B(1), Liu Y(1), Chen B(1).<br />
Author information: (1)Key Laboratory of Phytochemical R&D of Hunan Province and Key Laboratory of Chemical Biology and Traditional Chinese Medicine Research (Ministry of Education of China), Hunan Normal University, Changsha, 410081, China. (2)Yiyang Medical College, Yiyang 413000, China.<br />
Nano-sized particles are known to interfere with drug-metabolizing cytochrome P450 (CYP) enzymes, which can be anticipated to be a potential source of unintended adverse reactions, but the mechanisms underlying the inhibition are still not well understood. Herein we report a systematic investigation of the impacts of gold nanoparticles (AuNPs) on five major CYP isozymes under in vitro incubations of human liver microsomes (HLMs) with tannic acid (TA)-stabilized AuNPs in the size range of 5 to 100 nm. It is found that smaller AuNPs show more pronounced inhibitory effects on CYP2C9, CYP2C19, CYP2D6, and CYP3A4 in a dose-dependent manner, while 1A2 is the least susceptible to the AuNP inhibition. The size- and dose-dependent CYP-specific inhibition and the nonspecific drug-nanogold binding in the coincubation media can be significantly reduced by increasing the concentration ratio of microsomal proteins to AuNPs, probably via a noncompetitive mode. Remarkably, AuNPs are also found to exhibit a slow time-dependent inactivation of 2D6 and 3A4 in a β-nicotinamide adenine dinucleotide 2'-phosphate reduced tetrasodium salt hydrate (NADPH)-independent manner. During microsomal incubations, UV-vis spectroscopy, dynamic light scattering, and zeta-potential measurements were used to monitor the changes in particle properties under the miscellaneous AuNP/HLM/CYP dispersion system. An improved stability of AuNPs by mixing HLM with the gold nanocolloid reveals that the stabilization via AuNP-HLM interactions may occur on a faster time scale than the salt-induced nanoaggregation by incubation in phosphate buffer. The results suggest that the AuNP induced CYP inhibition can be partially attributed to its adhesion onto the enzymes to alter their structural conformations or onto the HLM membrane therefore impairing the integral membrane proteins. Additionally, AuNPs likely block the substrate pocket on the CYP surface, depending on both the particle characteristics and the structural diversity of the isozymes. These findings may represent additional mechanisms for the differential inhibitory effects arising from the coincubated AuNPs on the metabolic activities of the hepatic CYP isozymes.<br />
DOI: 10.1186/1556-276X-9-642 PMCID: PMC4266508 PMID: 25520592
|