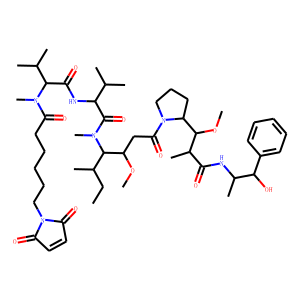
| InChI | InChI=1S/C49H78N6O10/c1-13-32(6)44(37(64-11)29-41(59)54-28-20-23-36(54)46(65-12)33(7)47(61)50-34(8)45(60)35-21-16-14-17-22-35)53(10)49(63)42(30(2)3)51-48(62)43(31(4)5)52(9)38(56)24-18-15-19-27-55-39(57)25-26-40(55)58/h14,16-17,21-22,25-26,30-34,36-37,42-46,60H,13,15,18-20,23-24,27-29H2,1-12H3,(H,50,61)(H,51,62)/t32-,33+,34+,36-,37+,42-,43-,44-,45+,46+/m0/s1 |
| Reference | 1. Mol Pharm. 2015 Jun 1;12(6):1863-71. doi: 10.1021/mp500666j. Epub 2015 Feb 9.
<br>
Site-Specific Conjugation of Monomethyl Auristatin E to Anti-CD30 Antibodies
Improves Their Pharmacokinetics and Therapeutic Index in Rodent Models.
<br>
Lhospice F(1), Brégeon D(1), Belmant C(1), Dennler P(2), Chiotellis A(3), Fischer
E(2), Gauthier L(1), Boëdec A(1), Rispaud H(1), Savard-Chambard S(1), Represa
A(1), Schneider N(1), Paturel C(1), Sapet M(1), Delcambre C(1), Ingoure S(1),
Viaud N(1), Bonnafous C(1), Schibli R(2)(3), Romagné F(4).
<br>
Author information: <br>
(1)†Innate Pharma SA, F13276 Marseille, France.
(2)‡Center for Radiopharmaceutical Sciences, ETH-PSI-USZ, Paul Scherrer
Institute, 5232 Villigen, Switzerland.
(3)§Institute of Pharmaceutical Sciences, Department of Chemistry and Applied
Biosciences, ETH Zurich, 8093 Zurich, Switzerland.
(4)∥MI-mAbs (C/0 CIML), Parc Scientifique et Technologique de Luminy, Avenue de
Luminy case 906, F13288 Marseille Cedex 9, France.
<br>
Antibody-drug conjugates (ADCs) have demonstrated clinical benefits that have led
to the recent FDA approval of KADCYLA and ADCETRIS. Most ADCs that are currently
in clinical use or development, including ADCETRIS, are produced by chemical
conjugation of a toxin via either lysine or cysteine residues, inevitably leading
to heterogeneous products with variable drug-to-antibody ratios (DARs). Here, we
describe the in vitro and in vivo characterization of four novel ADCs that are
based on the anti-CD30 antibody cAC10, which has the same polypeptide backbone as
ADCETRIS, and compare the results with the latter. Bacterial transglutaminase
(BTG) was exploited to site-specifically conjugate derivatives of monomethyl
auristatin E (all comprising a cleavable linker) to the glutamine at positions
295 and 297 of cAC10, thereby yielding homogeneous ADCs with a DAR of 4. In vitro
cell toxicity experiments using two different CD30-positive cell lines (Karpas
299 and Raji-CD30(+)) revealed comparable EC50 values for ADCETRIS (1.8 ± 0.4 and
3.6 ± 0.6 ng/mL, respectively) and the four cAC10-based ADCs (2.0 ± 0.4 to 4.9 ±
1.0 ng/mL). Quantitative time-dependent in vivo biodistribution studies (3-96 h
p.i.) in normal and xenografted (Karpas 299 cells) SCID mice were performed with
a selected (125)I-radioiodinated cAC10 ADC and compared with that of
(125)I-ADCETRIS. The chemo-enzymatically conjugated, radioiodinated ADC showed
higher tumor uptake (17.84 ± 2.2% ID/g 24 h p.i.) than (125)I-ADCETRIS (10.5 ±
1.8% ID/g 24 h p.i.). Moreover, (125)I-ADCETRIS exhibited higher nontargeted
liver and spleen uptake. In line with these results, the maximum tolerated dose
of the BTG-coupled ADC (>60 mg/kg) was significantly higher than that of ADCETRIS
(18 mg/kg) in rats. These results suggest that homogeneous ADCs display improved
pharmacokinetics and better therapeutic indexes compared to those of chemically
modified ADCs with variable DARs.
|