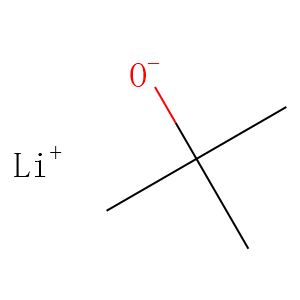| Reference | <p style=”box-sizing: border-box; margin: 0px 0px 10px; color: rgb(118, 118, 118); font-family: Roboto, "Helvetica Neue", Helvetica, Arial, sans-serif; font-size: 14px; font-variant-ligatures: normal; orphans: 2; widows: 2;”>
<span style=”color:#000000;”><span style=”font-size:12px;”><span style=”font-family:arial,helvetica,sans-serif;”><span style=”font-variant-ligatures: normal;”>1.Liang, Yu-Feng, et al. "Lithium tert-butoxide mediated α-alkylation of ketones with primary alcohols under transition-metal-free conditions." </span><i style=”font-family: Arial, sans-serif; font-size: 13px; font-variant-ligatures: normal;”>RSC advances</i><span style=”font-variant-ligatures: normal;”> 3.21 (2013): 7739-7742.</span><br />
2.Lee, C. W.; Han, S. J.; Virgil, S. C.; Stoltz, B. M. Stereochemical evaluation of bis(phosphine) copper catalysts for the asymmetric alkylation of 3-bromooxindoles with alpha-arylated malonate esters. <em style=”box-sizing: border-box;”>Tetrahedron </em>2015,<em style=”box-sizing: border-box;”>71</em> (22), 3666-3670.</span></span></span><span style=”font-family: arial, helvetica, sans-serif; font-size: 12px; color: rgb(0, 0, 0);”>3.Lou, S.; Ramirez, A.; Conlon, D. A. Catalytic syn-Selective Direct Aldol Reactions of Benzophenone Glycine Imine with Aromatic, Heteroaromatic and Aliphatic Aldehydes. </span><em style=”font-family: arial, helvetica, sans-serif; font-size: 12px; color: rgb(0, 0, 0); box-sizing: border-box;”>Adv. Synth. Catal.</em><span style=”font-family: arial, helvetica, sans-serif; font-size: 12px; color: rgb(0, 0, 0);”> </span><span style=”font-family: arial, helvetica, sans-serif; font-size: 12px; color: rgb(0, 0, 0);”>2015, </span><em style=”font-family: arial, helvetica, sans-serif; font-size: 12px; color: rgb(0, 0, 0); box-sizing: border-box;”>357</em><span style=”font-family: arial, helvetica, sans-serif; font-size: 12px; color: rgb(0, 0, 0);”> (1), 28-34.</span></p>
|