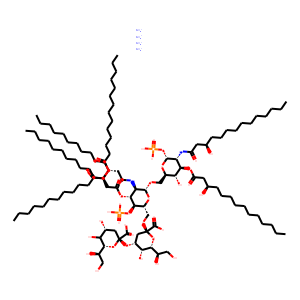
| InChI | InChI=1S/C110H202N2O39P2.4H3N/c1-7-13-19-25-31-37-38-44-50-56-62-68-92(123)142-82(66-60-54-48-42-35-29-23-17-11-5)72-94(125)146-104-96(112-90(121)71-81(65-59-53-47-41-34-28-22-16-10-4)141-91(122)67-61-55-49-43-36-30-24-18-12-6)105(144-88(102(104)150-152(133,134)135)78-140-109(107(129)130)74-86(98(127)101(148-109)85(119)76-114)147-110(108(131)132)73-83(117)97(126)100(149-110)84(118)75-113)139-77-87-99(128)103(145-93(124)70-80(116)64-58-52-46-40-33-27-21-15-9-3)95(106(143-87)151-153(136,137)138)111-89(120)69-79(115)63-57-51-45-39-32-26-20-14-8-2;;;;/h79-88,95-106,113-119,126-128H,7-78H2,1-6H3,(H,111,120)(H,112,121)(H,129,130)(H,131,132)(H2,133,134,135)(H2,136,137,138);4*1H3/t79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,95-,96-,97?,98-,99-,100-,101-,102-,103-,104-,105-,106-,109-,110-;;;;/m1..../s1 |
| Reference | 1: Sims K, Haynes CA, Kelly S, Allegood JC, Wang E, Momin A, Leipelt M, Reichart D, Glass CK, Sullards MC, Merrill AH Jr. Kdo2-lipid A, a TLR4-specific agonist, induces de novo sphingolipid biosynthesis in RAW264.7 macrophages, which is essential for induction of autophagy. J Biol Chem. 2010 Dec 3;285(49):38568-79. doi: 10.1074/jbc.M110.170621. Epub 2010 Sep 27. PMID: 20876532; PMCID: PMC2992289.
2: Crittenden CM , Morrison LJ , Fitzpatrick MD , Myers AP , Novelli ET , Rosenberg J , Akin LD , Srinivasa V , Shear JB , Brodbelt JS . Towards mapping electrostatic interactions between Kdo2-lipid A and cationic antimicrobial peptides via ultraviolet photodissociation mass spectrometry. Analyst. 2018 Jul 23;143(15):3607-3618. doi: 10.1039/c8an00652k. PMID: 29968868; PMCID: PMC6056329.
3: Chen WW, Chao YJ, Chang WH, Chan JF, Hsu YH. Phosphatidylglycerol Incorporates into Cardiolipin to Improve Mitochondrial Activity and Inhibits Inflammation. Sci Rep. 2018 Mar 20;8(1):4919. doi: 10.1038/s41598-018-23190-z. PMID: 29559686; PMCID: PMC5861085.
4: Dinasarapu AR, Gupta S, Ram Maurya M, Fahy E, Min J, Sud M, Gersten MJ, Glass CK, Subramaniam S. A combined omics study on activated macrophages–enhanced role of STATs in apoptosis, immunity and lipid metabolism. Bioinformatics. 2013 Nov 1;29(21):2735-43. doi: 10.1093/bioinformatics/btt469. Epub 2013 Aug 26. PMID: 23981351; PMCID: PMC3799469.
5: Gregus AM, Buczynski MW, Dumlao DS, Norris PC, Rai G, Simeonov A, Maloney DJ, Jadhav A, Xu Q, Wei SC, Fitzsimmons BL, Dennis EA, Yaksh TL. Inhibition of spinal 15-LOX-1 attenuates TLR4-dependent, nonsteroidal anti-inflammatory drug- unresponsive hyperalgesia in male rats. Pain. 2018 Dec;159(12):2620-2629. doi: 10.1097/j.pain.0000000000001373. PMID: 30130298; PMCID: PMC6237621.
6: Zhang G, Zhao L, Zhu J, Feng Y, Wu X. Anti-inflammatory activities and glycerophospholipids metabolism in KLA-stimulated RAW 264.7 macrophage cells by diarylheptanoids from the rhizomes of Alpinia officinarum. Biomed Chromatogr. 2018 Feb;32(2). doi: 10.1002/bmc.4094. Epub 2017 Oct 2. PMID: 28906002.
7: Chang WH, Ting HC, Chen WW, Chan JF, Hsu YH. Omega-3 and omega-6 fatty acid differentially impact cardiolipin remodeling in activated macrophage. Lipids Health Dis. 2018 Aug 28;17(1):201. doi: 10.1186/s12944-018-0845-y. PMID: 30153842; PMCID: PMC6114728.
8: Maurya MR, Gupta S, Li X, Fahy E, Dinasarapu AR, Sud M, Brown HA, Glass CK, Murphy RC, Russell DW, Dennis EA, Subramaniam S. Analysis of inflammatory and lipid metabolic networks across RAW264.7 and thioglycolate-elicited macrophages. J Lipid Res. 2013 Sep;54(9):2525-42. doi: 10.1194/jlr.M040212. Epub 2013 Jun 17. PMID: 23776196; PMCID: PMC3735949.
9: Kihara Y, Gupta S, Maurya MR, Armando A, Shah I, Quehenberger O, Glass CK, Dennis EA, Subramaniam S. Modeling of eicosanoid fluxes reveals functional coupling between cyclooxygenases and terminal synthases. Biophys J. 2014 Feb 18;106(4):966-75. doi: 10.1016/j.bpj.2014.01.015. PMID: 24559999; PMCID: PMC3945033.
10: Oishi Y, Hayashi S, Isagawa T, Oshima M, Iwama A, Shimba S, Okamura H, Manabe I. Bmal1 regulates inflammatory responses in macrophages by modulating enhancer RNA transcription. Sci Rep. 2017 Aug 1;7(1):7086. doi: 10.1038/s41598-017-07100-3. PMID: 28765524; PMCID: PMC5539165.
|