| Reference | 1.<span style="font-family: Arial, sans-serif; font-size: 13px; font-variant-ligatures: normal; orphans: 2; widows: 2;">Umezawa, Toshiaki. "Diversity in lignan biosynthesis." </span><i style="font-family: Arial, sans-serif; font-size: 13px; font-variant-ligatures: normal; orphans: 2; widows: 2;">Phytochemistry Reviews</i><span style="font-family: Arial, sans-serif; font-size: 13px; font-variant-ligatures: normal; orphans: 2; widows: 2;"> 2.3 (2003): 371-390.<br />
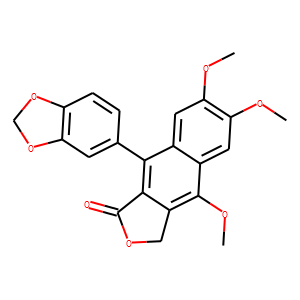
2.</span>J Asian Nat Prod Res. 2012;14(4):322-6. doi: 10.1080/10286020.2011.653561.Total synthesis of 6'-hydroxyjusticidin A.Xiong L(1), Bi MG, Wu S, Tong YF.
<div>
</div>
<div>
Author information:</div>
<div>
(1)State Key Laboratory of Bioactive Substance and Function of Natural Medicines, Department of New Drug Research and Development, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, </div>
<div>
Beijing 100050, China.</div>
<div>
</div>
<div>
The first total synthesis of 6'-hydroxyjusticidin A, isolated from Justicia procumbens L. with good inhibitory activity against cancer cells, has been accomplished. The structure was confirmed by ¹H NMR, ¹³C NMR, and HR-ESI-MS. The key steps involved a Diels-Alder cycloaddition reaction and a reduction in NaBH₄.</div>
<div>
</div>
<div>
DOI: 10.1080/10286020.2011.653561</div>
<div>
PMID: 22375869 [Indexed for MEDLINE]<br />
<br />
3.<span style="font-family: Arial, sans-serif; font-size: 13px; font-variant-ligatures: normal; orphans: 2; widows: 2;">Fukamiya, Narihiko, and Kuo-Hsiung Lee. "Antitumor agents, 81. Justicidin-A and diphyllin, two cytotoxic principles from Justicia procumbens." </span><i style="font-family: Arial, sans-serif; font-size: 13px; font-variant-ligatures: normal; orphans: 2; widows: 2;">Journal of Natural Products</i><span style="font-family: Arial, sans-serif; font-size: 13px; font-variant-ligatures: normal; orphans: 2; widows: 2;"> 49.2 (1986): 348-350.</span></div>
|