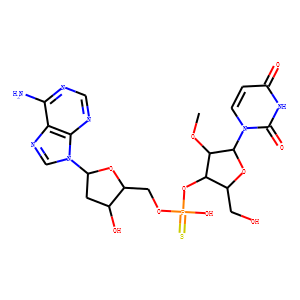
| InChI | InChI=1S/C20H26N7O10PS/c1-33-16-15(10(5-28)36-19(16)26-3-2-12(30)25-20(26)31)37-38(32,39)34-6-11-9(29)4-13(35-11)27-8-24-14-17(21)22-7-23-18(14)27/h2-3,7-11,13,15-16,19,28-29H,4-6H2,1H3,(H,32,39)(H2,21,22,23)(H,25,30,31)/t9-,10+,11+,13+,15+,16+,19+,38?/m0/s1 |
| Reference | 1. Antimicrob Agents Chemother. 2004 Jun;48(6):2318-20. <br />
Anti-hepatitis B virus activity of ORI-9020, a novel phosphorothioate
dinucleotide, in a transgenic mouse model. <br />
Iyer RP(1), Roland A, Jin Y, Mounir S, Korba B, Julander JG, Morrey JD. <br />
Author information: <br />
(1)Origenix Techonologies, Inc., St. Laurent, Quebec, Canada. <br />
ORI-9020, a novel dinucleotide, evaluated in transgenic mice expressing hepatitis
B virus (HBV), significantly reduced liver HBV DNA (P <!–= 0.001). Levels of HBeAg
and HBsAg in serum and of HBcAg in liver were not affected by treatment. A
minimal effective dosage was determined to be between 1.6 and 0.5 mg/kg of body
weight/day, which was similar to that observed for adefovir dipivoxil. <br /–> 2. Antimicrob Agents Chemother. 2004 Jun;48(6):2199-205. <br />
Phosphorothioate di- and trinucleotides as a novel class of anti-hepatitis B
virus agents. <br />
Iyer RP(1), Jin Y, Roland A, Morrey JD, Mounir S, Korba B. <br />
Author information: <br />
(1)Origenix Technologies, Inc., St.Laurent, Quebec, Canada. <br />
Several nucleoside analogs are under clinical development for use against
hepatitis B virus (HBV). Lamivudine (3TC), a nucleoside analog, and adefovir
dipivoxil (ADV), an acyclonucleotide analog, are clinically approved. However,
long-term treatment can induce viral resistance, and following the cessation of
therapy, viral rebound is frequently observed. There continues to be a need for
new antiviral agents with novel mechanisms of action. A library of more than 600
di- and trinucleotide compounds synthesized by parallel synthesis using a
combinatorial strategy was screened for potential inhibitors of HBV replication
using the chronically HBV-producing cell line 2.2.15. Through an iterative
process of synthesis, lead optimization, and screening, three analogs were
identified as potent inhibitors of HBV replication: dinucleotides ORI-7246 (drug
concentration at which a 10-fold reduction of HBV DNA was observed [EC(90)], 1.4
microM) and ORI-9020 (EC(90), 1.2 microM) and trinucleotide ORI-7170 (EC(90), 7.2
microM). These analogs inhibited the replication of both strands of HBV DNA. No
suppression of HBV protein synthesis or intracellular core particle formation by
these analogs was observed. No inhibition of HBV DNA strand elongation by the
analogs or their 5/’-triphosphate versions was apparent in in vitro polymerase
assays. Although the exact mechanism of action is not yet identified, present
data are consistent with an inhibition of the HBV reverse transcriptase-directed
priming step prior to elongation of the first viral DNA strand. In
transient-transfection assays, these analogs inhibited the replication of
3TC-resistant HBV. Synergistic interactions in combination treatments between the
analogs and either 3TC or ADV were observed. These compounds represent a novel
class of anti-HBV molecules and warrant further investigation as potential
therapeutic agents. <br />
|