| Reference | 1. Mol Pharmacol. 2010 Sep;78(3):419-30. doi: 10.1124/mol.110.065508. Epub 2010 Jun
1.
<br>
Cellular and pharmacological selectivity of the peroxisome proliferator-activated
receptor-beta/delta antagonist GSK3787.
<br>
Palkar PS(1), Borland MG, Naruhn S, Ferry CH, Lee C, Sk UH, Sharma AK, Amin S,
Murray IA, Anderson CR, Perdew GH, Gonzalez FJ, Müller R, Peters JM.
<br>
Author information: <br>
(1)Department of Veterinary and Biomedical Sciences and the Center for Molecular
Toxicology and Carcinogenesis, the Pennsylvania State University, University
Park, Pennsylvania 16802, USA.
<br>
The availability of high-affinity agonists for peroxisome proliferator-activated
receptor-beta/delta (PPARbeta/delta) has led to significant advances in our
understanding of the functional role of PPARbeta/delta. In this study, a new
PPARbeta/delta antagonist,
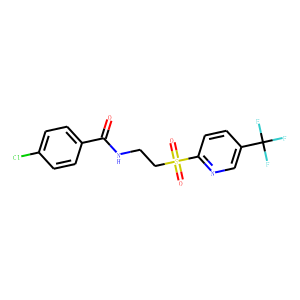
4-chloro-N-(2-{[5-trifluoromethyl)-2-pyridyl]sulfonyl}ethyl)benzamide (GSK3787),
was characterized using in vivo and in vitro models. Orally administered GSK3787
caused antagonism of
4-[2-(3-fluoro-4-trifluoromethyl-phenyl)-4-methyl-thiazol-5-ylmethylsulfanyl]-2-m
ethyl-phenoxy}-acetic acid (GW0742)-induced up-regulation of Angptl4 and Adrp
mRNA expression in wild-type mouse colon but not in Pparbeta/delta-null mouse
colon. Chromatin immunoprecipitation (ChIP) analysis indicates that this
correlated with reduced promoter occupancy of PPARbeta/delta on the Angptl4 and
Adrp genes. Reporter assays demonstrated antagonism of PPARbeta/delta activity
and weak antagonism and agonism of PPARgamma activity but no effect on PPARalpha
activity. Time-resolved fluorescence resonance energy transfer assays confirmed
the ability of GSK3787 to modulate the association of both PPARbeta/delta and
PPARgamma coregulator peptides in response to ligand activation, consistent with
reporter assays. In vivo and in vitro analysis indicates that the efficacy of
GSK3787 to modulate PPARgamma activity is markedly lower than the efficacy of
GSK3787 to act as a PPARbeta/delta antagonist. GSK3787 antagonized GW0742-induced
expression of Angptl4 in mouse fibroblasts, mouse keratinocytes, and human cancer
cell lines. Cell proliferation was unchanged in response to either GW0742 or
GSK3787 in human cancer cell lines. Results from these studies demonstrate that
GSK3787 can antagonize PPARbeta/delta in vivo, thus providing a new strategy to
delineate the functional role of a receptor with great potential as a therapeutic
target for the treatment and prevention of disease.
<br>
2. J Med Chem. 2010 Feb 25;53(4):1857-61. doi: 10.1021/jm900464j.
<br>
Identification and characterization of
4-chloro-N-(2-{[5-trifluoromethyl)-2-pyridyl]sulfonyl}ethyl)benzamide (GSK3787),
a selective and irreversible peroxisome proliferator-activated receptor delta
(PPARdelta) antagonist.
<br>
Shearer BG(1), Wiethe RW, Ashe A, Billin AN, Way JM, Stanley TB, Wagner CD, Xu
RX, Leesnitzer LM, Merrihew RV, Shearer TW, Jeune MR, Ulrich JC, Willson TM.
<br>
Author information: <br>
(1)Department of Metabolic Chemistry, Metabolic Diseases Centre of Excellence for
Drug Discovery, GlaxoSmithKline, 5 Moore Drive, Research Triangle Park, North
Carolina 27709, USA. [email protected]
<br>
4-Chloro-N-(2-{[5-trifluoromethyl)-2-pyridyl]sulfonyl}ethyl)benzamide 3 (GSK3787)
was identified as a potent and selective ligand for PPARdelta with good
pharmacokinetic properties. A detailed binding study using mass spectral analysis
confirmed covalent binding to Cys249 within the PPARdelta binding pocket. Gene
expression studies showed that pyridylsulfone 3 antagonized the transcriptional
activity of PPARdelta and inhibited basal CPT1a gene transcription. Compound 3 is
a PPARdelta antagonist with utility as a tool to elucidate PPARdelta cell biology
and pharmacology.
|