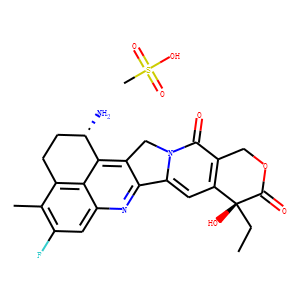
| Synonyms | DX 8951; DX-8951; DX8951.;(1S,9S)-1-amino-9-ethyl-5-fluoro-9-hydroxy-4-methyl-1,2,3,9,12,15-hexahydro-10H,13H-benzo[de]pyrano[3/’,4/’:6,7]indolizino[1,2-b]quinoline-10,13-dione methanesulfonic acid
|
| Reference | 1. Cancer. 2003 Sep 1;98(5):900-7.
<br>
A Phase II study of intravenous exatecan mesylate (DX-8951f) administered daily
for 5 days every 3 weeks to patients with metastatic breast carcinoma.
<br>
Esteva FJ(1), Rivera E, Cristofanilli M, Valero V, Royce M, Duggal A, Colucci P,
DeJager R, Hortobagyi GN.
<br>
Author information: <br>
(1)Department of Breast Medical Oncology, University of Texas M. D. Anderson
Cancer Center, 1515 Holcombe Boulevard, Box 424, Houston, TX 77030, USA.
[email protected]
<br>
BACKGROUND: The objective of the current study was to determine the antitumor
activity, safety, and pharmacokinetic (PK) profile of exatecan mesylate in
patients with anthracycline-resistant and taxane-resistant, metastatic breast
carcinoma.<br>
METHODS: All patients had clinical evidence of metastatic breast carcinoma;
disease resistance or progression after chemotherapy that included anthracyclines
and taxanes; no prior chemotherapy with camptothecin derivatives; and
bidimensionally measurable disease. The starting dose of exatecan mesylate was
either 0.5 mg/m(2) per day or 0.3 mg/m(2) per day, depending on prior
chemotherapy exposure. PK blood samples were collected from each patient during
the first course of therapy.<br>
RESULTS: Thirty-nine patients received a total of 172 courses of therapy (median,
4 courses; range, 1-16 courses). Three patients (7.7%) had a partial response,
and 20 patients (51.3%) had either a minor response or stable disease.
Approximately 20% of patients had stable disease for 6 months or longer. The
median time to disease progression was 3 months, and the median survival was 14
months. The most frequent severe adverse event was neutropenia. The most frequent
severe (Grade 3-4) nonhematologic toxicities were fatigue, nausea, headache,
myalgia, constipation, emesis, and paresthesias in 28%, 10%, 10%, 8%, 8%, 5%, and
5% of patients, respectively. Exatecan mesylate displayed linear PK
characteristics at the doses administered. The average plasma clearance, total
volume of distribution, and terminal elimination half-life were approximately 1.4
L per hour per m(2), 12 L/m(2), and 8 hours, respectively.
CONCLUSIONS: Exatecan mesylate had moderate activity in patients with
anthracycline-refractory and taxane-refractory, metastatic breast carcinoma. The
toxicity profile of exatecan mesylate was acceptable, and it appeared to have
linear PK characteristics on the basis of multiple dose administration.
<br>
2. Ann Oncol. 2003 Jun;14(6):913-21.
<br>
Phase I and pharmacokinetic study of the topoisomerase I inhibitor, exatecan
mesylate (DX-8951f), using a weekly 30-minute intravenous infusion, in patients
with advanced solid malignancies.
<br>
Braybrooke JP(1), Boven E, Bates NP, Ruijter R, Dobbs N, Cheverton PD, Pinedo HM,
Talbot DC.
<br>
Author information: <br>
(1)Cancer Research UK Medical Oncology Unit, Churchill Hospital, Oxford, UK.
<br>
BACKGROUND: The topoisomerase I inhibitor exatecan mesylate (DX-8951f ) is a
water-soluble hexacyclic analogue of camptothecin that does not require enzymatic
activation. This study determined the toxicity, maximum tolerated dose (MTD),
pharmacokinetics and pharmacodynamics of a weekly intravenous (i.v.) schedule of
DX-8951f.<br>
PATIENTS AND METHODS: Thirty-five patients with advanced solid malignancies,
stratified as minimally (MP) or heavily (HP) pre-treated, received escalating
doses of DX-8951f as 30-min i.v. infusions for three out of every 4 weeks.
Pharmacokinetics were described after the first infusion of DX-8951f.<br>
RESULTS: Infusions (244) of DX-8951f were administered with a median of two
cycles (range 1-10). The main toxicity observed was haematological. There was no
significant gastrointestinal toxicity. Two patients (6%) had confirmed partial
responses. Twelve patients (39%) had stable disease. DX-8951f had a terminal
elimination half-life of approximately 8 h and a clearance of 2 l/h/m(2). The
area under the plasma concentration versus time curve (AUC( infinity )) and the
maximum plasma concentration (C(max)) increased linearly with the dose. A linear
relationship was present for the percentage decrease in neutrophil counts or
platelet counts and AUC( infinity ) as well as C(max).<br>
CONCLUSIONS: The dose-limiting toxicity of DX-8951f is neutropenia for MP
patients and neutropenia and thrombocytopenia for HP patients. Evidence for
clinical activity was seen, suggesting phase II study of the drug is indicated.
Using this schedule the recommended dose is 2.75 mg/m(2)/week for MP patients and
2.10 mg/m(2)/week for HP patients.
<br>
|