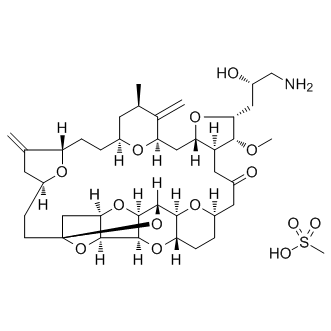
| IUPAC Name | (1S,3S,6S,9S,12S,14R,16R,18S,20R,21R,22S,26R,29S,31R,32S,33R,35R,36S)-20-[(2S)-3-amino-2-hydroxypropyl]-21-methoxy-14-methyl-8,15-dimethylidene-2,19,30,34,37,39,40,41-octaoxanonacyclo[24.9.2.13,32.13,33.16,9.112,16.018,22.029,36.031,35]hentetracontan-24-one;methanesulfonic acid |
| InChI | InChI=1S/C40H59NO11.CH4O3S/c1-19-11-24-5-7-28-20(2)12-26(45-28)9-10-40-17-33-36(51-40)37-38(50-33)39(52-40)35-29(49-37)8-6-25(47-35)13-22(42)14-27-31(16-30(46-24)21(19)3)48-32(34(27)44-4)15-23(43)18-41;1-5(2,3)4/h19,23-39,43H,2-3,5-18,41H2,1,4H3;1H3,(H,2,3,4)/t19-,23+,24+,25-,26+,27+,28+,29+,30-,31+,32-,33-,34-,35+,36+,37+,38-,39+,40+;/m1./s1 |
| Reference | 1. Expert Rev Anticancer Ther. 2014 Jun;14(6):649-65. doi: 10.1586/14737140.2014.920693.<br />
Eribulin mesylate in the management of metastatic breast cancer and other solid cancers: a drug review.<br />
Polastro L(1), Aftimos PG, Awada A.<br />
Author information:<br />
(1)Medical Oncology Clinic, Institut Jules Bordet, Université Libre de Bruxelles , Brussels , Belgium.<br />
In the new era of /'precision/' cancer medicine, new drug development has shifted from cytotoxic chemotherapy to molecularly targeted agents. Eribulin mesylate, a microtubule-destabilizing agent, is the only /'classical/' cytotoxic agent approved for the treatment of breast cancer in the last 7 years. This synthetic analogue of halichondrin B, isolated from the marine sponge /'Halicondria Okaida/', was responsible for prolonging overall survival of heavily pretreated metastatic breast cancer patients in a large Phase III trial. Eribulin is now under clinical development in earlier settings such as the neo-adjuvant and adjuvant settings. Furthermore, its unique mechanism of action and the absence of cross-resistance with taxanes have led to the design of clinical trials in multiple indications: bladder cancer, lung cancer, prostate cancer… The main adverse events are neutropenia, fatigue and peripheral neuropathy.<br />
2. Cancer Treat Rev. 2012 Apr;38(2):143-51. doi: 10.1016/j.ctrv.2011.03.006. Epub 2011 May 8.<br />
Eribulin mesylate, a novel microtubule inhibitor in the treatment of breast cancer.<br />
Cortes J(1), Montero AJ, Glück S.<br />
Author information:<br />
(1)Department of Oncology, Vall d′Hebron University Hospital, Barcelona, Spain. [email protected]<br />
BACKGROUND: Microtubule-targeted agents are one of the most common classes of chemotherapeutic drug for the treatment of breast cancer. Limitations of current microtubule-targeted agents such as primary or secondary resistance of cancer cells and side effects like neuropathy prompted the discovery and introduction of newer more effective drugs. This review aims to provide a summary of the novel halichondrin B analog eribulin mesylate (E7389) and illustrate where it is placed in the treatment arena versus other agents that are approved or are currently in various stages of clinical development.<br />
METHODS: Preclinical and clinical trial (phases I-III) data for eribulin were obtained from scientific journals and meeting abstracts, posters, and oral presentations. The use of current and other emerging microtubule inhibiting agents in breast cancer was also surveyed and briefly reviewed. RESULTS: Eribulin mesylate at a dose of 1.4 mg/m(2) given on days 1 and 8 of a 21-day cycle increased overall survival in patients with metastatic breast cancer (MBC). Neutropenia, fatigue, alopecia, nausea and anemia were common adverse events (AEs) associated with eribulin in clinical studies. A low incidence of peripheral neuropathy was also associated with eribulin in clinical studies (21-26%). Other emerging microtubule targeted agents, such as vinflunine and larotaxel, also reported efficacy in patients with MBC who had received prior chemotherapy, with grade 3/4 neutropenia being the most common AEs for both agents.<br />
CONCLUSIONS: Eribulin mesylate offers clinical activity in advanced breast cancer through improved overall survival, its favorable side-effect profile and convenience of preparation and administration.<br />
3. Adv Ther. 2011 Nov;28(11):973-85. doi: 10.1007/s12325-011-0070-9. Epub 2011 Oct 19.<br />
Survival benefit of eribulin mesylate in heavily pretreated metastatic breast cancer: what next?<br />
Aftimos P(1), Awada A.<br />
Author information:<br />
(1)Medical Oncology Clinic, Institut Jules Bordet, 121 Boulevard de Waterloo, 1000, Brussels, Belgium. [email protected]<br />
Eribulin is a synthetic analog of halichondrin B, a non-taxane microtubule inhibitor extracted from the marine sponge, Halichondria okaida. It presents a novel mechanism of action and is active on cancer cells resistant to other antimicrotubule agents. It was granted approval in the USA and Europe for the treatment of heavily pretreated metastatic breast cancer. Early trials had shown activity in this setting with main toxicities being neutropenia and neuropathy in patients where quality of life is essential. Approvals were granted after a phase 3 trial demonstrated overall survival benefit in metastatic breast cancer previously treated with anthracyclines, taxanes, and capecitabine, in a setting where most treatments are failing to demonstrate a survival benefit. Recent data suggest that this survival benefit is also consistent in the elderly with no excess toxicity. Future strategies of eribulin are being tested in ongoing trials, evaluating this drug in earlier metastatic lines as well as in the adjuvant and the neoadjuvant settings. 4. Expert Opin Pharmacother. 2010 Jun;11(9):1587-93. doi: 10.1517/14656566.2010.486790.<br />
Eribulin mesylate for the treatment of breast cancer.<br />
Cigler T(1), Vahdat LT.<br />
Author information:<br />
(1)Division of Hematology and Medical Oncology, Weill Medical College of Cornell University, 425 East 61st Street – 8th Floor, New York, NY 10021, USA. [email protected]<br />
IMPORTANCE OF THE FIELD: Breast cancer is the most common cause of cancer-related death among women in the USA, and additional effective and well-tolerated chemotherapeutic agents are urgently needed. Eribulin mesylate (E7389), a synthetic analog of the marine macrolide halichondrin B, is a microtubule inhibitor with a unique tubulin binding site and mechanism of action. AREAS COVERED IN THIS REVIEW: Based on a review of the literature between 2005 and 2010, we present a summary of eribulin and its clinical activity, specifically in metastatic breast cancer.<br />
WHAT THE READER WILL GAIN: The mechanism of action of eribulin, preclinical data indicating antitumor activity of eribulin and data from Phase I and II clinical trials evaluating the efficacy and tolerability of eribulin are presented. TAKE HOME MESSAGE: Based on data from Phase I and II clinical trials, we conclude that eribulin seems to have efficacy in metastatic breast cancer, even among women with heavily pretreated and taxane-resistant disease. In addition, eribulin has a manageable side-effect profile, consisting mainly of neutropenia and fatigue, and most notably a low incidence of peripheral neuropathy. With these encouraging results, additional Phase II and III studies are ongoing. Eribulin seems to be a promising new agent for the treatment of metastatic breast cancer.<br />
|