| Overview of Clinical Research | <span style=”font-family:arial,helvetica,sans-serif;”><span style=”font-size:12px;”><span style=”color:#000000;”>Entecavir is a <span style=”font-variant-ligatures: normal; orphans: 2; widows: 2;”>DNA-directed DNA polymerase inhibitor. </span><span style=”font-variant-ligatures: normal; orphans: 2; widows: 2;”>Phase III development is ongoing in South Korea for Hepatitis B (In children, In adolescents) (PO) (NCT01079806).</span></span></span></span>
|
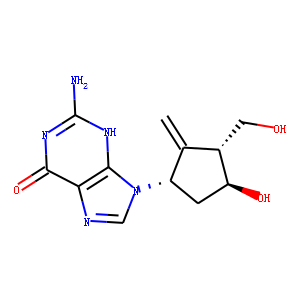
| InChI | InChI=1S/C12H15N5O3/c1-5-6(3-18)8(19)2-7(5)17-4-14-9-10(17)15-12(13)16-11(9)20/h4,6-8,18-19H,1-3H2,(H3,13,15,16,20)/t6-,7-,8-/m0/s1 |
| Reference | 1. Curr Med Res Opin. 2005 Nov;21(11):1845-56.<br />
A review of entecavir in the treatment of chronic hepatitis B infection.<br />
Rivkin A(1).<br />
Author information:<br />
(1)Arnold and Marie Schwartz College of Pharmacy and Health Sciences, Long Island University, Brooklyn, NY 11201, USA. [email protected]<br />
BACKGROUND: Infection with the hepatitis B virus (HBV) affects two billion people worldwide, and an estimated 400 million people are chronically infected. Currently, FDA-approved regimens for the treatment of chronic HBV include interferon-alpha2b, peginterferon-alpha2a, lamivudine, adefovir dipivoxil, and recently, entecavir.<br />
OBJECTIVE: The purpose of this review is to evaluate the pharmacokinetic and pharmacodynamic properties, and the clinical efficacy and safety of entecavir in the treatment of nucleoside-naĩve and nucleoside-resistant HBeAg-positive and HBeAg-negative chronic hepatitis B (CHB). SEARCH METHODOLOGY: Computerized searches of PubMed and International Pharmaceutical Abstracts from 1985 to July 10, 2005, were performed with the search headings: entecavir, BMS-200475, and chronic hepatitis B.<br />
FINDINGS: Entecavir, a new deoxyguanosine analog, represents a third agent within the nucleoside/nucleotide HBV polymerase inhibitor class with distinct advantages over lamivudine and adefovir dipivoxil: it has a three-step mechanism of action, is the most potent inhibitor of HBV DNA polymerase, is not associated with any major adverse effects, and has a limited potential for resistance. In phase II and III clinical trials, entecavir was found to be superior to lamivudine for all primary endpoints evaluated in both nucleoside-naïve and lamivudine-resistant patients. Entecavir was effective in both HBeAg-positive and HBeAg-negative nucleoside-naïve patients. At this time, optimal duration of entecavir therapy is unknown.<br />
CONCLUSION: Entecavir represents a new first- or second-line treatment option for patients chronically infected with HBV. Long-term efficacy and safety studies as well as studies of entecavir in combination with interferon products or other nucleoside/nucleotide analogs are eagerly awaited.<br />
<br />
2. Expert Opin Investig Drugs. 2003 Apr;12(4):683-8.<br />
Entecavir: a potent new antiviral drug for hepatitis B.<br />
Honkoop P(1), De Man RA.<br />
Author information:<br />
(1)Department of Gastroenterology and Hepatology, Erasmus Medical Centre, University Hospital, Rotterdam, The Netherlands.<br />
Entecavir, a new deoxyguanine nucleoside analogue, is a selective inhibitor of the replication of the hepatitis B virus. In vitro this compound has proven to be far more effective than other nucleoside analogues. In animal models, an impressive reduction of serum viral DNA has been observed with covalently closed circular DNA and hepatitis B viral core antigen negativity in liver biopsy specimens. In clinical studies, entecavir revealed excellent suppression of hepatitis B virus replication without significant side effects or evidence of mitochondrial toxicity. Until now, no entecavir-resistant viral mutants have been described. Prolonged therapy as well as prophylactic therapy, for example, in liver transplant recipients, is feasible and not limited by breakthrough infections. Data on entecavir therapy for treatment of nucleoside-naive, wild-type hepatitis B virus is being generated in Phase III clinical trials worldwide for both hepatitis B envelope antigen-positive and -negative subpopulations, as well as in lamivudine-resistant patients. Based on mechanism and potency of interferon and entecavir, clinical trials with combination therapy are eagerly awaited.<br />
|