| Reference | [1]. J Exp Clin Cancer Res. 2021 Mar 19;40(1):106. doi: 10.1186/s13046-021-01907-9.<br />
MZ1 co-operates with trastuzumab in HER2 positive breast cancer.<br />
Noblejas-López MDM(#)(1)(2), Nieto-Jiménez C(#)(3), Galán-Moya EM(2)(4), Tebar-García D(1)(2), Montero JC(3)(5)(6), Pandiella A(3)(5)(6)(7), Burgos M(8)(9), Ocaña A(10)(11)(12).<br />
Author information: (1)Translational Research Unit, Translational Oncology Laboratory, Albacete University Hospital, C/Francisco Javier de Moya esquina C/Laurel, Albacete, Spain. (2)Centro Regional de Investigaciones Biomédicas, Castilla-La Mancha University (CRIB-UCLM), Albacete, Spain. (3)Instituto de Biología Molecular y Celular del Cáncer (IBMCC-CIC), Salamanca, Spain. (4)Faculty of Nursing, Castilla-La Mancha University (UCLM), Albacete, Spain. (5)Instituto de Investigación Biomédica de Salamanca (IBSAL), Salamanca, Spain. (6)CIBERONC, Salamanca, Spain. (7)Consejo Superior de Investigaciones Científicas (CSIC), Salamanca, Spain. (8)Translational Research Unit, Translational Oncology Laboratory, Albacete University Hospital, C/Francisco Javier de Moya esquina C/Laurel, Albacete, Spain. [email protected]. (9)Centro Regional de Investigaciones Biomédicas, Castilla-La Mancha University (CRIB-UCLM), Albacete, Spain. [email protected]. (10)Translational Research Unit, Translational Oncology Laboratory, Albacete University Hospital, C/Francisco Javier de Moya esquina C/Laurel, Albacete, Spain. [email protected]. (11)Centro Regional de Investigaciones Biomédicas, Castilla-La Mancha University (CRIB-UCLM), Albacete, Spain. [email protected]. (12)Experimental Therapeutics Unit, Medical Oncology Department, Hospital Clínico San Carlos (HCSC), Instituto de Investigación Sanitaria (IdISSC) and CIBERONC, Calle Del Prof Martín Lagos, s/n, 28040, Madrid, Spain. [email protected]. (#)Contributed equally<br />
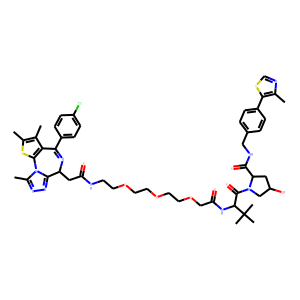
BACKGROUND: Although the anti-HER2 antibody trastuzumab augments patient survival in HER2+ breast cancer, a relevant number of patients progress to this treatment. In this context, novel drug combinations are needed to increase its antitumor activity. In this work, we have evaluated the efficacy of proteolysis targeting chimera (PROTAC) compounds based on BET inhibitors (BETi) to augment the activity of trastuzumab in HER2+ breast cancer models. METHODS: BT474 and SKBR3 HER2+ breast cancer cell lines were used. The effects of trastuzumab and the BET-PROTAC MZ1 either alone or in combination, were evaluated using MTT proliferation assays, three-dimensional invasion and adhesion cultures, flow cytometry, qPCR and Western blot. In vivo studies were carried out in a xenografted model in mice. Finally, a Clariom_S_Human transcriptomic array was applied to identify deregulated genes after treatments. RESULTS: MZ1 induced a higher antiproliferative effect compared to the BETi JQ1. The combination of MZ1 and -trastuzumab significantly decreased cell proliferation, the formation of three-dimensional structures and cellular invasion compared to either of the drugs alone. Evaluation of apoptosis resulted in an increase of cell death following treatment with the combination, and biochemical studies displayed modifications of apoptosis and DNA damage components. In vivo administration of agents alone or combined, to tumors orthotopically xenografted in mice, resulted in a decrease of the tumor volume only after MZ1-Trastuzumab combination treatment. Results from a transcriptomic array indicated a series of newly described transcription factors including HOXB7, MEIS2, TCERG1, and DNAJC2, that were associated to poor outcome in HER2+ breast cancer subtype and downregulated by the MZ1-trastuzumab combination. CONCLUSIONS: We describe an active novel combination that includes the BET-PROTAC MZ1 and trastuzumab, in HER2+ tumors. Further studies should be performed to confirm these findings and pave the way for their future clinical development.<br />
DOI: 10.1186/s13046-021-01907-9 PMCID: PMC7980639 PMID: 33741018<br />
<br />
[2]. Pharmaceutics. 2020 Oct 19;12(10):986. doi: 10.3390/pharmaceutics12100986.<br />
Controlled Delivery of BET-PROTACs: In Vitro Evaluation of MZ1-Loaded Polymeric Antibody Conjugated Nanoparticles in Breast Cancer.<br />
Cimas FJ(1)(2), Niza E(3)(4), Juan A(2)(3), Noblejas-López MDM(1)(2), Bravo I(3)(4), Lara-Sanchez A(5), Alonso-Moreno C(3)(4), Ocaña A(1)(2)(6).<br />
Author information: (1)Oncología Traslacional, Centro Regional de Investigaciones Biomédicas, 02008 Albacete, Spain. (2)Oncología Traslacional, Unidad de Investigación del Complejo Hospitalario Universitario de Albacete, 02008 Albacete, Spain. (3)Centro Regional de Investigaciones Biomédicas, Unidad NanoCRIB, 02008 Albacete, Spain. (4)Facultad de Farmacia de Albacete, Universidad de Castilla-La Mancha, 02008 Albacete, Spain. (5)Facultad de Ciencias y Tecnologías Químicas, Universidad de Castilla-La Mancha, 13005 Ciudad Real, Spain. (6)Experimental Therapeutics Unit, Hospital clínico San Carlos, IdISSC and CIBERONC, 28029 Madrid, Spain.<br />
Bromo and extraterminal domain (BET) inhibitors-PROteolysis TArgeting Chimera (BETi-PROTAC) is a new family of compounds that induce proteasomal degradation through the ubiquitination of the tagged to BET inhibitors Bromodomain proteins, BRD2 and BRD. The encapsulation and controlled release of BET-PROTACs through their vectorization with antibodies, like trastuzumab, could facilitate their pharmacokinetic and efficacy profile. Antibody conjugated nanoparticles (ACNPs) using PROTACs have not been designed and evaluated. In this pioneer approach, the commercial MZ1 PROTAC was encapsulated into the FDA-approved polymeric nanoparticles. The nanoparticles were conjugated with trastuzumab to guide the delivery of MZ1 to breast tumoral cells that overexpress HER2. These ACNPs were characterized by means of size, polydispersity index, and Z-potential. Morphology of the nanoparticles, along with stability and release studies, completed the characterization. MZ1-loaded ACNPs showed a significant cytotoxic effect maintaining its mechanism of action and improving its therapeutic properties.<br />
DOI: 10.3390/pharmaceutics12100986 PMCID: PMC7589709 PMID: 33086530<br />
<br />
[3]. Eur J Cell Biol. 1999 Feb;78(2):134-42. doi: 10.1016/s0171-9335(99)80015-6.<br />
Analysis of NO synthase expression in neuronal, astroglial and fibroblast-like derivatives differentiating from PCC7-Mz1 embryonic carcinoma cells.<br />
Gath I(1), Steppuhn A, Maelicke A, Reinhardt S, Förstermann U.<br />
Author information: (1)Department of Pharmacology, Johannes Gutenberg University, Mainz/Germany.<br />
We studied the expression of the NO synthase isoforms in an in vitro model of neural development using RT-PCR, Western blot and immunohistochemistry. Murine PCC7-Mz1 cells (Jostock et al., Eur. J. Cell Biol. 76, 63-76, 1998) differentiate in the presence of all-trans retinoic acid and dibutyryl cAMP along the neural pathway into neuron-like, fibroblast-like and astroglia-like cells. Undifferentiated cells showed immunofluorescent staining for neuronal-type NOS I and endothelial-type NOS III. This expression pattern was retained in those cells differentiating into neurofilament- and tau protein-positive neuronal cells. Thymocyte alloantigen (Thy1.2/CD 90.2)-positive fibroblasts, appearing around day 3, and glial fibrillary acidic protein (GFAP)-positive astroglial cells, appearing after day 6 of differentiation, stained negative for any NOS isoform. Starting at day 6 of differentiation, expression of inducible-type NOS II could be stimulated with cytokines in a subset of cells, which may represent activated astrocytes. NOS II was always undetectable in non-induced cultures. These data indicate that the ability of stem cells to express NOS I and NOS III is only retained when the cells differentiate along the neuronal lineage, while a small subpopulation of cells acquires the ability to express NOS II in response to cytokines.<br />
DOI: 10.1016/s0171-9335(99)80015-6 PMID: 10099936 [Indexed for MEDLINE]<br />
<br />
[4]. Arch Virol. 2008;153(10):1855-65. doi: 10.1007/s00705-008-0201-z. Epub 2008 Sep 20.<br />
Isolation, characterization and genome sequencing of phage MZTP02 from Bacillus thuringiensis MZ1.<br />
Liao W(1), Song S, Sun F, Jia Y, Zeng W, Pang Y.<br />
Author information: (1)State Key Laboratory of Biocontrol, School of Life Sciences, Institute of Entomology, Sun Yat-sen Zhongshan University, Guangzhou, People's Republic of China. [email protected]<br />
A lysogenic phage, MZTP02, was produced via induction by mitomycin C from Bacillus thuringiensis (B. thuringiensis) strain MZ1. Plaques were about 3 mm in diameter with a small inner zone consisting of new B. thuringiensis colonies. Electron microscopic analysis showed that MZTP02 had a long tail (220 nm x 18 nm) and an icosahedral head (82 nm x 85 nm). MZTP02 was insensitive to organic solvents such as chloroform, and infected six B. thuringiensis strains. Its complete genome contained 15,717 base pairs (bp) with 37.55% G + C content. Two inverted terminal repeats consisting of 40 bp were 65% identical. Twenty putative open reading frames (ORFs) were found in the MZTP02 genome, and nine predicted proteins, including two terminase subunits, portal protein, minor head protein, scaffold protein, two putative membrane proteins, tail component, and minor structural protein, showed similarity to other phage proteins. But six ORFs were unique. The presence of a terminal protein at the 5'-terminus was demonstrated using proteinase K, lambda exonuclease and E. coli exonuclease III to digest the genome DNA. A TMP phylogenetic tree was constructed based on amino acid sequences from ten phages.<br />
DOI: 10.1007/s00705-008-0201-z PMID: 18807113 [Indexed for MEDLINE]<br />
<br />
[5]. Wei Sheng Wu Xue Bao. 2007 Feb;47(1):92-7.<br />
[Biology of two lysogenic phages from Bacillus thuringiensis MZ1].<br />
[Article in Chinese]<br />
Liao W(1), Sun F, Song SY, Shi W, Pang Y.<br />
Author information: (1)State Key Laboratory of Biocontrol, Sun Yat- Sen Zhongshan University, Guangzhou 510275, China. [email protected]<br />
A Bacillus thuringiensis (Bt) fermentative strain MZ1 (subsp. kurstaki) , from a company in Meixian County of Guangdong Province, produce toxins during sporulation and are extensively used in the field to control pest insects (Lepidoptera) in China. But some unknown or random factors that inhibited or stopped B. t growth in the fermentation can be regarded as reflecting the exist of lysogenic phage. Therefore, strain MZI and its lysogenic phages were studied in this paper. With indicator strain ZK1, two kind of phage plaques, one with about 3mm diameter and the other with about 1mm diameter, can be observed after strain MZ1 cultured in plates or flasks was induced by mitomycin C. Then, two lysogenic phages, namely MZTP01 and MZTP02, were isolated and characterized in biology. They belonged to family Siphoviridae, which had icosahedral heads (MZTP01 :82nm x 85nm; MZTP02: 75nm x 55nm) and long tails (MZTP01: 220nm x 18nm; MZTP02: 183nm x 12nm) without flexibility. Host range examination showed that six and seven (including indicator strain ZKl) out of 113 B. t strains saved in our laboratory were sensitive to MZTP01 and MZTP02, respectively. MZTP01 was more stable than MZTP02 against pH value, ultraviolet and heat treatment, but contrary against organic solvents. For MZTP01 and MZTP02, K values in the neutralization reactions were 45 and 326, respectively. Both phages had no relationship in their antigenicity. Burst size of phage MZTP02 was 175, two times more than that of MZTP01. Latent time of MZTP02 was 40min, one times shorter than that of MZTP01. It was suggested that both phages DNA be linear dsDNA through the typical absorption curves, reaction with diphenylamine, DNase sensitivity and acridine orange staining. And this was in good accord with the previous findings that all tailed phages being dsDNA moleculars. The genomic DNA and their restriction maps showed that both molecular weights should be between 9.4 – 23kb. Both phage genomic DNAs were digested by Hind III, producing eight and nine bands, respectively. The results above show that strain MZl is proved a double lysogen. It is considered to be the main reason of great losses in the production. The biological information of the two lysogenic phages belonging to strain MZ1 is provided to help solve the problem and the two lysogenic phages is prepared for complete DNA sequencing in the next step.<br />
PMID: 17436632 [Indexed for MEDLINE]
|