| Reference | [1]. Eur J Paediatr Dent. 2019 Sep;20(3):224-232. doi: 10.23804/ejpd.2019.20.03.11.<br />
Biological effects of resin monomers on oral cell populations: descriptive analysis of literature.<br />
Pagano S(1), Coniglio M(1), Valenti C(1), Negri P(1), Lombardo G(1), Costanzi E(2), Cianetti S(1), Montaseri A(3), Marinucci L(2).<br />
Author information: (1)Department of Biomedical and Surgical Sciences, Odontostomatological University Centre: Chair Prof. Stefano Cianetti, University of Perugia, Perugia, Italy. (2)Department of Experimental Medicine, Section of Biosciences and Medical Embryology, University of Perugia, Perugia, Italy. (3)Anatomical Sciences Department, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran.<br />
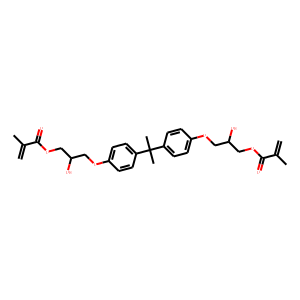
AIM: Recently, the application of restorative materials containing metacrilate monomers in the conservative and paediatric dentistry has focused on the possible negative effects due to the use of these composites. In particular the release of monomers from reconstructions as a result of an insufficient polymerisation, can spread along the mucosal and dental tissues with potential immunological ed cytotoxic effects. Regarding to the importance of this issue, the aim of this study is to provide a descriptive review of the literature on potential local and systemic interactions of metacrylic and acrylic monomers with the immune system, both in vitro and in vivo. RESULTS: The most highly used monomers in composite materials applied in conservative dentistry include: 2-hydroessietil- methacrylate (HEMA), triethylene glycol-dimethacrylate (TEGDMA), bisphenol A glycidyl-methacrylate (BisGMA) and urethane- dimethacrylate (UDMA). Different investigations have been performed for better understanding of the potential side effects of metacrylic monomers on immune system cells. Different factors such as cell population, exposure time and parameters more strictly connected to these materials, such as molecular weight, chemical composition and mechanical characteristics, seem to be directly involved in these reactions.<br />
DOI: 10.23804/ejpd.2019.20.03.11 PMID: 31489823 [Indexed for MEDLINE]<br />
<br />
[2]. Acta Biomater. 2013 Feb;9(2):5088-99. doi: 10.1016/j.actbio.2012.10.002. Epub 2012 Oct 8.<br />
Biological responses of silver-coated thermosets: an in vitro and in vivo study.<br />
Marsich E(1), Travan A, Donati I, Turco G, Kulkova J, Moritz N, Aro HT, Crosera M, Paoletti S.<br />
Author information: (1)Department of Life Sciences, University of Trieste, Via Giorgieri 5, Trieste I-34127, Italy. [email protected]<br />
Bisphenol A glycidylmethacrylate (BisGMA)/triethyleneglycol dimethacrylate (TEGDMA) thermosets are biomaterials commonly employed for orthopedic and dental applications; for both these fields, bacterial adhesion to the surface of the implant represents a major issue for the outcome of the surgical procedures. In this study, the antimicrobial properties of a nanocomposite coating formed by polysaccharide 1-deoxylactit-1-yl chitosan (Chitlac) and silver nanoparticles (nAg) on methacrylate thermosets were studied. The Chitlac-nAg system showed good anti-bacterial and anti-biofilm activity although its biocidal properties can be moderately, albeit significantly, inhibited by serum proteins. In vitro studies on the silver release kinetic in physiological conditions showed a steady metal release associated with a gradual loss of antimicrobial activity. However, after 3weeks there was still effective protection against bacterial colonization which could be accounted for by the residual silver. This time-span could be considered adequate to confer short-term protection from early peri-implant infections. Preliminary in vivo tests in a mini-pig animal model showed good biological compatibility of Chitlac-nAg-coated materials when implanted in bony tissue. The comparison was made with implants of titanium Ti6Al4V alloy and with a Chitlac-coated thermoset. Bone healing patterns and biocompatibility parameters observed for nAg-treated material were comparable with those observed for control implants.<br />
DOI: 10.1016/j.actbio.2012.10.002 PMID: 23059413 [Indexed for MEDLINE]<br />
<br />
[3]. J Dent. 2005 Jan;33(1):49-55. doi: 10.1016/j.jdent.2004.08.001.<br />
In vitro embryotoxicity assessment with dental restorative materials.<br />
Schwengberg S(1), Bohlen H, Kleinsasser N, Kehe K, Seiss M, Walther UI, Hickel R, Reichl FX.<br />
Author information: (1)Axiogenesis AG, Joseph-Stelzmann-Str. 50, 50931 Köln, Germany.<br />
OBJECTIVES: Resin (co)monomers may be released from restorative dental materials and can diffuse into the tooth pulp or the gingiva, and can reach the saliva and the circulating blood. Genotoxic potential of some dental composite components has been clearly documented. The genotoxic effects of xenobiotics can represent a possible step in tumor initiation and/or embryotoxicity/teratogenesis. A modified fluorescent mouse embryonic stem cell test (R.E.Tox) was used to test the embryotoxic potential of following dental restorative materials: Bisphenol A glycidylmethacrylate (BisGMA), urethanedimethacrylate (UDMA), hydroxyethylmethacrylate (HEMA), and triethyleneglycoldimethacrylate (TEGDMA), as well as some of their metabolic intermediates 2,3-epoxy-2-methyl-propionicacid-methylester (EMPME), methacrylic acid (MA), and 2,3-epoxy-2-methylpropionic acid (EMPA). METHODS: Mouse embryonic stem (ES) cells stably transfected with a vector containing the gene for the green fluorescent protein under control of the cardiac alpha-myosin heavy chain promoter were differentiated in the presence of various concentrations of the test compounds for 12 days. Fluorescence was measured using the TECAN Safire and values were expressed as percent of control values. To distinguish between cytotoxic and embryotoxic effects, all compounds were tested in a standard MTT assay. RESULTS: HEMA, TEGDMA and EMPME did not influence the differentiation process of ES cells towards cardiac myocytes. No cytotoxic effects were observed at any of the concentration levels tested. Exposure to BisGMA resulted in a 50% decrease in cell survival and a very strong inhibition of cell differentiation at 10(-5)M (p<0.01). Embryotoxic effects were also present at 10(-6) and 10(-7)M (p<0.05). EMPA induced a decrease in ES cell differentiation at 10(-5)M (p<0.01) without cytotoxic effects. No embryotoxic effects were induced at lower concentrations. Exposure to UDMA resulted in a slight decrease of cell differentiation at 10(-5)M (p<0.05). Exposure of cells to MA resulted in an increase of cardiac differentiation up to 150% (p<0.05) at 10(-5)M without cytotoxic effects. CONCLUSIONS: BisGMA induced a significant high embryotoxic/teratogenic effect over a large range of concentration. Therefore attention should be focused on this dental monomer, which should be investigated further by in vivo experiments.<br />
DOI: 10.1016/j.jdent.2004.08.001 PMID: 15652168 [Indexed for MEDLINE]<br />
<br />
[4]. Acta Biomater. 2011 Jan;7(1):337-46. doi: 10.1016/j.actbio.2010.07.024. Epub 2010 Jul 23.<br />
Silver-polysaccharide nanocomposite antimicrobial coatings for methacrylic thermosets.<br />
Travan A(1), Marsich E, Donati I, Benincasa M, Giazzon M, Felisari L, Paoletti S.<br />
Author information: (1)Department of Life Sciences, University of Trieste, Italy. [email protected]<br />
Bisphenol A glycidylmethacrylate (BisGMA)/triethyleneglycol dimethacrylate (TEGDMA) thermosets are receiving increasing attention as biomaterials for dental and orthopedic applications; for both these fields, bacterial adhesion to the surface of the implant represents a major issue for the outcome of the surgical procedure. Moreover, the biological behaviour of these materials is influenced by their ability to establish proper interactions between their surface and the eukaryotic cells of the surrounding tissues, which is important for good implant integration. The aim of this work was to develop an antimicrobial non-cytotoxic coating for methacrylic thermosets by means of a nanocomposite material based on a lactose-modified chitosan and antibacterial silver nanoparticles. The coating was characterized by UV-vis spectrophotometry, optical microscopy, transmission electron microscopy (TEM) and scanning electron microscopy (SEM). In vitro tests were employed for a biological characterization of the material: antimicrobial efficacy tests were carried out with both Gram+ and Gram- strains. Osteoblast-like cell-lines, primary human fibroblasts and adipose-derived stem cells, were used for LDH cytotoxicity assays and Alamar blue cell proliferation assays. Cell morphology and distribution were evaluated by SEM and confocal laser scanning microscopy. In vitro results showed that the nanocomposite coating is effective in killing both bacterial strains and that this material does not exert any significant cytotoxic effect towards tested cells, which are able to firmly attach and proliferate on the surface of the coating. Such biocompatible antimicrobial polymeric films containing silver nanoparticles may have good potential for surface modification of medical devices, especially for prosthetic applications in orthopedics and dentistry.<br />
DOI: 10.1016/j.actbio.2010.07.024 PMID: 20656078 [Indexed for MEDLINE]<br />
<br />
[5]. Biomacromolecules. 2010 Mar 8;11(3):583-92. doi: 10.1021/bm9011419.<br />
Surface modification and polysaccharide deposition on BisGMA/TEGDMA thermoset.<br />
Travan A(1), Donati I, Marsich E, Bellomo F, Achanta S, Toppazzini M, Semeraro S, Scarpa T, Spreafico V, Paoletti S.<br />
Author information: (1)Department of Life Sciences, University of Trieste, Via Licio Giorgieri 1, 34127 – Trieste, Italy. [email protected]<br />
Bisphenol A glycidylmethacrylate (BisGMA)/triethyleneglycol dimethacrylate (TEGDMA) thermosets and composites are well-known examples of biomaterials for dental applications that are receiving growing interest for orthopedic applications. While mechanical bulk properties are guaranteed by the presence of reinforcing fibers, in vitro and in vivo performances of these materials are ultimately driven by their ability to establish proper interactions between their surface and the surrounding tissues. Hence, the development of novel chemical processes enabling the introduction of bioactive molecules on the surface of these methacrylate-based thermosets is of particular interest. In the present work, we have devised a chemical strategy to expose carboxylic groups on the surface of the BisGMA/TEGDMA thermoset. The presence of negative charges was confirmed by Fourier transform infrared-attenuated total reflectance and by UV-vis spectrophotometry. Bulk mechanical properties and surface morphology of the thermoset were only slightly affected upon chemical functionalization. The activated material was further refined by the deposition of a lactose-modified chitosan (chitlac) driven by strong electrostatic interactions. The presence of the bioactive polysaccharide was confirmed by fluorescence spectroscopy and by confocal laser scanning microscopy measurements. Scratch tests were performed to evaluate the mechanical behavior of the coating. Finally, in vitro tests revealed that the presence of chitlac led to a slight enhancement of cell proliferation with respect to the unmodified BisGMA/TEGDMA thermoset. This effect was more pronounced when chitlac decorated with an arginine-glycine-aspartic acid (RGD) peptide was used in the preparation of the coating. In the latter case, the in vitro performance of the coated BisGMA/TEGDMA thermoset became comparable with that of clinically used roughened titanium.<br />
DOI: 10.1021/bm9011419 PMID: 20158281 [Indexed for MEDLINE]
|