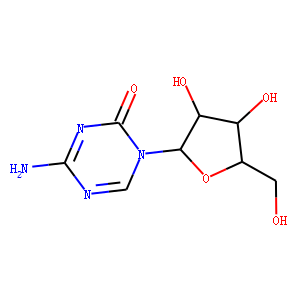
| Reference | [1]. Oncologist. 2015 Dec;20(12):1404-12. doi: 10.1634/theoncologist.2015-0165. Epub 2015 Oct 13.<br />
Oral Azacitidine (CC-486) for the Treatment of Myelodysplastic Syndromes and Acute Myeloid Leukemia.<br />
Cogle CR(1), Scott BL(2), Boyd T(3), Garcia-Manero G(4).<br />
Author information: (1)Division of Hematology and Oncology, Department of Medicine, College of Medicine, University of Florida, Gainesville, Florida, USA [email protected]. (2)Fred Hutchinson Cancer Research Center, Seattle, Washington, USA. (3)North Star Lodge Cancer Center, Yakima, Washington, USA. (4)Department of Leukemia, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.<br />
The myelodysplastic syndromes (MDS) comprise a heterogeneous group of clonal myeloid malignancies characterized by multilineage cytopenias, recurrent cytogenetic abnormalities, and risk of progression to acute myeloid leukemia (AML). AML, which can occur de novo as well as secondary to MDS, is characterized by malignant clones of myeloid lineage in the bone marrow and peripheral blood, with dissemination into tissues. The cytidine nucleoside analog and epigenetic modifier azacitidine is approved in the U.S. for the treatment of all French-American-British subtypes of MDS and in many countries for the treatment of AML with 20%-30% blasts and multilineage dysplasia according to the World Health Organization classification. Benefits of azacitidine treatment of patients with AML with >30% blasts have also been shown in a recent phase III trial. Oral administration of azacitidine may enhance patient convenience, eliminate injection-site reactions, allow for alternative dosing and scheduling, and enable long-term treatment. Phase I studies with oral azacitidine (CC-486) have shown biological activity, clinical responses, and tolerability in patients with MDS and AML. Extended dosing schedules of oral azacitidine (for 14 or 21 days of 28-day cycles) are currently under investigation as frontline therapy in patients with lower risk MDS, as maintenance therapy for patients with AML not eligible for stem cell transplant, and as maintenance therapy for patients with MDS or AML following stem cell transplant. This review presents clinical data supporting the use of injectable azacitidine in MDS and AML and examines the rationale for and results of the clinical development of oral azacitidine.<br />
DOI: 10.1634/theoncologist.2015-0165 PMCID: PMC4679081 PMID: 26463870 [Indexed for MEDLINE]<br />
<br />
[2]. IARC Monogr Eval Carcinog Risks Hum. 1990;50:47-63.<br />
Azacitidine.<br />
[No authors listed]<br />
PMCID: PMC7681440 PMID: 1705587 [Indexed for MEDLINE]<br />
<br />
[3]. Recent Results Cancer Res. 2010;184:159-70. doi: 10.1007/978-3-642-01222-8_11.<br />
5-Azacytidine/Azacitidine.<br />
Müller A(1), Florek M.<br />
Author information: (1)Division of Blood and Marrow Transplantation, Stanford University, School of Medicine, 269, West Campus Drive CCSR, Stanford, CA 94305, USA. [email protected]<br />
5-Azacytidine is a pyrimidine nucleoside analog that has been discovered more than 40 years ago. Despite remarkable responses in the treatment of acute myeloid leukemias in the 1970s no earlier than 2004 has this agent been approved by the US FDA for the treatment of all subtypes of myelodysplatic syndromes (MDS). For the first time a drug was proven to alter the natural course of MDS, as demonstrated in three clinical trials conducted by the CALG B. Complete remission rates ranged between 10-17%, and more recently, a significant survival benefit for MDS patients treated with 5-Azacytidine could be established. The antineoplastic activity is due to incorporation into RNA with disruption of RNA metabolism, and inhibition of DNA methylation.Strategies of combining epigenetic manipulation with other 'new' drugs aim at increasing the efficacy of the hypomethylating agents. Particularly histone deacetylase inhibitors have been deemed useful therapeutic partners, and preliminary results are promising.<br />
DOI: 10.1007/978-3-642-01222-8_11 PMID: 20072837 [Indexed for MEDLINE]<br />
<br />
[4]. Rep Carcinog. 2011;12:56-7.<br />
Azacitidine.<br />
National Toxicology Program.<br />
PMID: 21829253 [Indexed for MEDLINE]<br />
<br />
[5]. Nat Rev Drug Discov. 2005 Apr;4(4):275-6. doi: 10.1038/nrd1698.<br />
Azacitidine.<br />
Issa JP(1), Kantarjian HM, Kirkpatrick P.<br />
Author information: (1)Department of Leukemia, University of Texas MD Anderson Cancer Center, Houston, Texas 77030, USA. [email protected] <[email protected]><br />
DOI: 10.1038/nrd1698 PMID: 15861567 [Indexed for MEDLINE]</[email protected]>
|