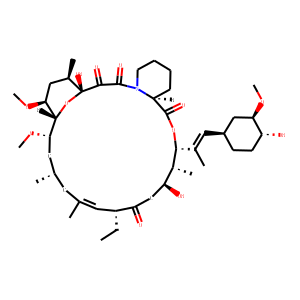
| IUPAC Name | (1R,9S,12S,13R,14S,17R,18E,21S,23S,24R,25S,27R)-17-ethyl-1,14-dihydroxy-12-[(E)-1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]prop-1-en-2-yl]-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo[22.3.1.04,9]octacos-18-ene-2,3,10,16-tetrone |
| Reference | 1. Appl Microbiol Biotechnol. 2017 Jun;101(11):4581-4592. doi:
10.1007/s00253-017-8242-4. Epub 2017 Mar 27.
<br><br>
Engineering of the LysR family transcriptional regulator FkbR1 and its target
gene to improve ascomycin production.
<br>
Song K(1)(2), Wei L(1)(2), Liu J(1)(2), Wang J(1)(2), Qi H(1)(2), Wen J(3)(4).
<br>
Author information: <br>
(1)Key Laboratory of System Bioengineering (Tianjin University), Ministry of
Education, Tianjin, 300072, People’s Republic of China.
(2)SynBio Research Platform, Collaborative Innovation Center of Chemical Science
and Engineering (Tianjin), School of Chemical Engineering and Technology, Tianjin
University, Tianjin, 300072, People’s Republic of China.
(3)Key Laboratory of System Bioengineering (Tianjin University), Ministry of
Education, Tianjin, 300072, People’s Republic of China. [email protected].
(4)SynBio Research Platform, Collaborative Innovation Center of Chemical Science
and Engineering (Tianjin), School of Chemical Engineering and Technology, Tianjin
University, Tianjin, 300072, People’s Republic of China. [email protected].
<br><br>
Ascomycin (FK520), a macrocyclic polyketide natural antibiotic, displays high
anti-fungal and immunosuppressive activity. In this study, the LysR family
transcriptional regulator FkbR1 was characterized, and its role in ascomycin
biosynthesis was explored by gene deletion, complementation, and overexpression.
Inactivation of fkbR1 led to 67.5% reduction of ascomycin production, which was
restored by complementation of fkbR1. Overexpression of fkbR1 resulted in a 33.5%
increase in ascomycin production compared with the parent strain FS35. These
findings indicated that FkbR1 was a positive regulator for ascomycin production.
Quantitative RT-PCR analysis revealed that the expressions of fkbE, fkbF, fkbS,
and fkbU were downregulated in the fkbR1 deletion strain and upregulated in the
fkbR1 overexpression strain. Electrophoretic mobility shift assays (EMSAs) in
vitro and chromatin immunoprecipitation (ChIP)-qPCR assays in vivo indicated that
FkbR1 bound to the intergenic region of fkbR1-fkbE. To investigate the roles of
the target genes fkbE and fkbF in ascomycin production, the deletion and
overexpressions of fkbE and fkbF were implemented, respectively. Overexpression
of fkbE resulted in a 45.6% increase in ascomycin production, but little change
was observed in fkbF overexpression strain. To further enhance ascomycin
production, the fkbR1 and fkbE combinatorial overexpression strain OfkbRE was
constructed with the ascomycin yield increased by 69.9% to 536.7 mg/L compared
with that of the parent strain. Our research provided a helpful strategy to
increase ascomycin production via engineering FkbR1 and its target gene.
<br><br>
2. Pharmacol Biochem Behav. 2006 Jul;84(3):511-6. Epub 2006 Jul 26.
<br><br>
Anticonvulsant effect of the calcineurin inhibitor ascomycin on seizures induced
by picrotoxin microperfusion in the rat hippocampus.
<br><br>
Vázquez-López A(1), Sierra-Paredes G, Sierra-Marcuño G.
<br><br>
Author information: <br>
(1)Neuroscience Division, Department of Biochemistry and Molecular Biology,
School of Medicine, University of Santiago, San Francisco 1, 15782 Santiago de
Compostela, Spain.
<br>
The potential in vivo anticonvulsant effect of calcineurin (protein phosphatase
2B) inhibitor ascomycin against seizures induced by intrahippocampal
microdialysis of picrotoxin was examined in the present study. After establishing
individual picrotoxin seizure thresholds, ascomycin was continually microperfused
into the rat hippocampus through microdialysis probes at concentrations 10, 50
and 100 microM. No behavioral or electroencephalographic effects were observed
during microperfusion of ascomycin alone. Low concentrations (10 microM) of
ascomycin did not prevent picrotoxin seizures, however, 50 and 100 microM
ascomycin showed antiepileptic effect, completely suppressing seizures in 41.7%
and 75% of the animals studied respectively. Mean seizure duration and mean
number of seizures were significantly reduced (P < 0.01) by microperfusion of 100
microM ascomycin. Calcineurin activity might be involved in the biochemical
changes leading to picrotoxin-induced epileptic seizures. The present findings
provide additional in vivo evidence of the involvement of
phosphorylation/dephosphorylation mechanisms in the development of epileptic
seizures, suggesting that calcineurin modulation may be a possible strategy in
the search for new anticonvulsant drugs.
<br><br>
3. Int J Mol Med. 2002 Feb;9(2):141-5.
<br><br>
Identification of genes coding enzymes for ascomycin tetra-hydropyranose ring
formation.
<br><br>
Uchida T(1), Fujimori F, Nagata N.
<br><br>
Author information: <br>
(1)Department of Pathology, Institute of Aging, Development and Cancer, Tohoku
University, Aoba, Sendai 980-8575, Japan. [email protected]
<br>
Macrocyclic polyketides have generated great interest in biosynthetic chemistry
because of the structural complexity and medicinal activities. The synthetic
genes consist of the number and type of active sites of modular polyketide
synthases. The cosmid library – prepared with the ascomycin (an antibiotics with
immunosuppressive activity) – producer, Streptomyces sp. AA6554 genome was
screened with an ascomycin ketosynthase gene probe, and one and a half modules
were isolated. Database analysis shows that one of the modules consists of the
genes coding a series of enzymes for the tetra-hydropyranose ring synthesis.
<br>
|