| Reference | 1. Br J Cancer. 2007 Sep 17;97(6):785-91.
<br>
PDK-1/AKT pathway as a novel therapeutic target in rhabdomyosarcoma cells using
OSU-03012 compound.
<br>
Cen L(1), Hsieh FC, Lin HJ, Chen CS, Qualman SJ, Lin J.
<br>
Author information: <br>
(1)Center for Childhood Cancer, Columbus Children’s Research Institute,
Department of Pediatrics, The Ohio State University, Columbus, OH 43205, USA.
<br>
Rhabdomyosarcoma (RMS) is the most common paediatric soft-tissue sarcoma
including two major subtypes, alveolar rhabdomyosarcoma (ARMS) and embryonal
rhabdomyosarcoma (ERMS). Increasing evidence suggests that oncogenesis of RMS
involves multiple stages of signalling protein dysregulation which may include
prolonged activation of serine/threonine kinases such as
phosphoinositide-dependent kinase-1 (PDK-1) and AKT. To date, whether PDK-1/AKT
pathway is activated in RMS is unknown. This study was to examine phosphorylation
status of AKT and to evaluate a novel small molecular inhibitor, OSU-03012
targeting PDK-1 in RMS. We examined phosphorylation levels of AKT using ARMS and
ERMS tissue microarray and immunohistochemistry staining. Our results showed
phospho-AKT(Thr308) level is elevated 42 and 35% in ARMS and ERMS, respectively.
Phospho-AKT(Ser473) level is also increased 43% in ARMS and 55% in ERMS.
Furthermore, we showed that OSU-03012 inhibits cell viability and induces
apoptosis in ARMS and ERMS cell lines (RH30, SMS-CTR), which express elevated
phospho-AKT levels. Normal cells are much less sensitive to OSU-03012 and in
which no detectable apoptosis was observed. This study showed, for the first
time, that PDK-1/AKT pathway is activated in RMS and may play an important role
in survival of RMS. PDK-1/AKT pathway may be an attractive therapeutic target for
cancer intervention in RMS using OSU-03012.
<br><br>
2. Clin Cancer Res. 2007 Aug 15;13(16):4750-8.
<br>
OSU-03012, a novel celecoxib derivative, is cytotoxic to myeloma cells and acts
through multiple mechanisms.
<br>
Zhang S(1), Suvannasankha A, Crean CD, White VL, Johnson A, Chen CS, Farag SS.
<br>
Author information: <br>
(1)Division of Hematology and Oncology, Department of Internal Medicine, Indiana
University School of Medicine, Indianapolis, Indiana 46202, USA.
<br>
PURPOSE: OSU-03012 is a novel celecoxib derivative, without cyclooxygenase-2
inhibitory activity, capable of inducing apoptosis in various cancer cells types,
and is being developed as an anticancer drug. We investigated the in vitro
activity of OSU-03012 in multiple myeloma (MM) cells.<br>
EXPERIMENTAL DESIGN: U266, ARH-77, IM-9, and RPMI-8226, and primary myeloma cells
were exposed to OSU-03012 for 6, 24, or 72 h. Cytotoxicity, caspase activation,
apoptosis, and effects on intracellular signaling pathways were assessed.
RESULTS: OSU-03012 was cytotoxic to MM cells with mean LC50 3.69 +/- 0.23 and
6.25 +/- 0.86 micromol/L and at 24 h for primary MM cells and cell lines,
respectively. As a known PDK-1 inhibitor, OSU-03012 inhibited the PI3K/Akt
pathway with downstream effects on BAD, GSK-3beta, FoxO1a, p70S6K, and MDM-2.
However, transfection of MM cells with constitutively active Akt failed to
protect against cell death, indicating activity against other pathways is
important. Phospho (p)-signal transducers and activators of transcription 3 and
p-MAP/ERK kinase 1/2 were down-regulated, suggesting that OSU-03012 also
inhibited the Janus-activated kinase 2/signal transducer and activator of
transcription 3 and mitogen-activated protein kinase pathways. Although
expression of Bcl-2 proteins was unchanged, OSU-03012 also down-regulated
survivin and X-linked inhibitor of apoptosis (XIAP), and also induced G2 cell
cycle arrest with associated reductions in cyclins A and B. Finally, although
OSU-03012 induced cleavage of caspases 3, 8 and 9, caspase inhibition did not
prevent cell death.<br>
CONCLUSIONS: We conclude that OSU-03012 has potent activity against MM cells and
acts via different mechanisms in addition to phosphoinositide-3-kinase/Akt
pathway inhibition. These studies provide rationale for the clinical
investigation of OSU-03012 in MM.
<br><br>
3. Mol Pharmacol. 2006 Aug;70(2):589-603. Epub 2006 Apr 18.
<br>
OSU-03012 promotes caspase-independent but PERK-, cathepsin B-, BID-, and
AIF-dependent killing of transformed cells.
<br>
Yacoub A(1), Park MA, Hanna D, Hong Y, Mitchell C, Pandya AP, Harada H, Powis G,
Chen CS, Koumenis C, Grant S, Dent P.
<br>
Author information: <br>
(1)Department of Biochemistry, Massey Cancer Center Virginia Commonwealth
University, Richmond, VA 23298-0058, USA.
<br>
We determined one mechanism by which the putative phosphoinositide-dependent
kinase (PDK)-1 inhibitor
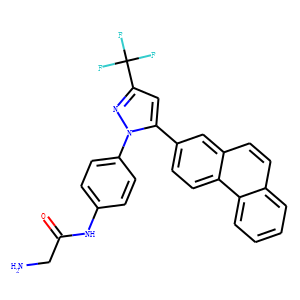
2-amino-N-{4-[5-(2-phenanthrenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]-phenyl}ace
tamide (OSU-03012) killed primary human glioma and other transformed cells.
OSU-03012 caused a dose-dependent induction of cell death that was not altered by
p53 mutation, expression of ERBB1 vIII, or loss of phosphatase and tensin homolog
deleted on chromosome 10 function. OSU-03012 promoted cell killing to a greater
extent in glioma cells than in nontransformed astrocytes. OSU-03012 and ionizing
radiation caused an additive, caspase-independent elevation in cell killing in
96-h viability assays and true radiosensitization in colony formation assays. In
a cell type-specific manner, combined exposure to OSU-03012 with a
mitogen-activated protein kinase kinase 1/2 inhibitor, phosphoinositide
3-kinase/AKT inhibitors, or parallel molecular interventions resulted in a
greater than additive induction of cell killing that was independent of AKT
activity and caspase function. OSU-03012 lethality as a single agent or when
combined with signaling modulators was not modified in cells lacking expression
of BIM or of BAX/BAK. OSU-03012 promoted the release of cathepsin B from the
lysosomal compartment and release of AIF from mitochondria. Loss of
BH3-interacting domain (BID) function, overexpression of BCL(XL), and inhibition
of cathepsin B function suppressed cell killing and apoptosis-inducing factor
(AIF) release from mitochondria. In protein kinase R-like endoplasmic reticulum
kinase-/- cells, the lethality of OSU-03012 was attenuated which correlated with
reduced cleavage of BID and with suppression of cathepsin B and AIF release into
the cytosol. Our data demonstrate that OSU-03012 promotes glioma cell killing
that is dependent on endoplasmic reticulum stress, lysosomal dysfunction, and
BID-dependent release of AIF from mitochondria, and whose lethality is enhanced
by irradiation or by inhibition of protective signaling pathways.
<br><br>
4. Blood. 2005 May 15;105(10):4021-7. Epub 2005 Jan 21.
<br>
Synergistic interactions between imatinib mesylate and the novel
phosphoinositide-dependent kinase-1 inhibitor OSU-03012 in overcoming imatinib
mesylate resistance.
<br>
Tseng PH(1), Lin HP, Zhu J, Chen KF, Hade EM, Young DC, Byrd JC, Grever M,
Johnson K, Druker BJ, Chen CS.
<br>
Author information: <br>
(1)Division of Medicinal Chemistry, College of Pharmacy, The Ohio State
University, 336 L. M. Parks Hall, Columbus, OH 43210, USA.
<br>
Resistance to the Ableson protein tyrosine (Abl) kinase inhibitor imatinib
mesylate has become a critical issue for patients in advanced phases of chronic
myelogenous leukemia. Imatinib-resistant tumor cells develop, in part, as a
result of point mutations within the Abl kinase domain. As protein kinase B (Akt)
plays a pivotal role in Abl oncogene-mediated cell survival, we hypothesize that
concurrent inhibition of Akt will sensitize resistant cells to the residual
apoptotic activity of imatinib mesylate, thereby overcoming the resistance. Here,
we examined the effect of OSU-03012, a celecoxib-derived
phosphoinositide-dependent kinase-1 (PDK-1) inhibitor, on imatinib
mesylate-induced apoptosis in 2 clinically relevant breakpoint cluster region
(Bcr)-Abl mutant cell lines, Ba/F3p210(E255K) and Ba/F3p210(T315I). The 50%
inhibitory concentration (IC50) values of imatinib mesylate to inhibit the
proliferation of Ba/F3p210(E255K) and Ba/F3p210(T315I) were 14 +/- 4 and 30 +/- 2
microM, respectively. There was no cross-resistance to OSU-03012 in these mutant
cells with an IC50 of 5 microM irrespective of mutations. Nevertheless, in the
presence of OSU-03012 the susceptibility of these mutant cells to
imatinib-induced apoptosis was significantly enhanced. This synergistic action
was, at least in part, mediated through the concerted effect on phospho-Akt.
Together these data provide a novel therapeutic strategy to overcome imatinib
mesylate resistance, especially with the Abl mutant T315I.
<br>
|