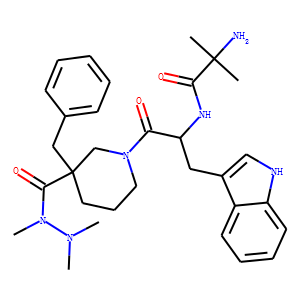
| InChI | 1S/C31H42N6O3/c1-30(2,32)28(39)34-26(18-23-20-33-25-15-10-9-14-24(23)25)27(38)37-17-11-16-31(21-37,29(40)36(5)35(3)4)19-22-12-7-6-8-13-22/h6-10,12-15,20,26,33H,11,16-19,21,32H2,1-5H3,(H,34,39)/t26-,31-/m1/s1 |
| Reference | 1. Support Care Cancer. 2017 May;25(5):1651-1659. doi: 10.1007/s00520-016-3560-0.
Epub 2017 Jan 10.
<br>
Anamorelin for cancer anorexia-cachexia syndrome: a systematic review and
meta-analysis.
<br>
Bai Y(1), Hu Y(1), Zhao Y(1), Yu X(1)(2), Xu J(1)(3), Hua Z(4), Zhao Z(5).
<br>
Author information: <br>
(1)The First College of Clinical Medicine, Nanjing University of Chinese
Medicine, Nanjing, China.
(2)Medical Research Central of First College of Clinical Medicine, Nanjing,
China.
(3)Affiliated Hospital of Nantong University, Nantong, China.
(4)Zhenjiang Hospital of Traditional Chinese Medicine, Zhenjiang, China.
(5)The First College of Clinical Medicine, Nanjing University of Chinese
Medicine, Nanjing, China. [email protected].
<br>
PURPOSE: The aim of this study was to evaluate the therapeutic effects of
Anamorelin on patients with cancer anorexia-cachexia syndrome (CACS) based on a
meta-analysis of published randomized trials.
METHODS: We searched PubMed, Embase, Medline, and the Cochrane Central Register
of Controlled Trials databases. Data from each selected study were evaluated
individually. All continuous outcomes were calculated by the mean difference or
standardized mean difference with 95% confidence interval for each study.
Heterogeneity was assessed by using the Chi2 test at a significance level of
P < 0.1, in addition to the I 2 statistic (I 2 > 50% indicated substantial
heterogeneity).
RESULT: At last, four studies were included from 284 records. In three studies,
lean body mass was reported and there was a significant difference between
placebo and Anamorelin groups (P < 0.00001), without significant heterogeneity (I
2 = 0%). All the four studies reported the body weight change from baseline, and
there was significant difference between placebo and Anamorelin groups
(P = 0.007), but with high heterogeneity (I 2 = 97%). Two studies reported
Anderson Symptom Assessment Scale (ASAS) score, and Anamorelin significantly
increased the total ASAS score of CACS patients (P < 0.00001), without any
heterogeneity (I 2 = 0%). Three studies reported non-dominant handgrip strength,
and there was no significant difference between Anamorelin and placebo groups
(P = 0.16). Three studies reported insulin-like growth factor-1 level, and there
was significant difference between Anamorelin and placebo groups (P = 0.02), but
with high heterogeneity (I 2 = 96%). Three studies reported IGF binding protein-3
concentration. Anamorelin significantly increased such concentration compared
with placebo did (P < 0.00001). However, there was still higher heterogeneity (I
2 = 59%). All the included studies reported adverse events. Compared with
placebo, Anamorelin induced fewer adverse events, but there was no significant
difference between the two groups (RR = 0.07, P = 0.35).
CONCLUSION: In the included studies, Anamorelin had some positive effects to
relieve the symptoms and improved the quality of life. However, the heterogeneity
was apparent, so the clinical effects of Anamorelin should be further validated
by increasing the sample size, varying the range of doses during treatment, and
observing other outcomes. We are still confident for the future application of
Anamorelin in phase III clinical trials.
|