| Reference | [1]. J Pharm Biomed Anal. 2010 Sep 21;53(1):24-8. doi: 10.1016/j.jpba.2010.03.008. Epub 2010 Mar 11.<br />
Isolation and structure elucidation of an interaction product of aminotadalafil found in an illegal health food product.<br />
Häberli A(1), Girard P, Low MY, Ge X.<br />
Author information: (1)Official Medicines Control Laboratory, Swissmedic, Hallerstrasse 7, CH-3000 Berne 9, Switzerland.<br />
An interaction product of aminotadalafil was isolated from an illegal health food product. The structure of the interaction product was elucidated by means of IR, NMR, and mass spectroscopy. The hitherto unknown compound was characterized as condensation product of aminotadalafil and hydroxymethylfuraldehyde and is probably the result of a drug-excipient incompatibility.<br />
DOI: 10.1016/j.jpba.2010.03.008 PMID: 20363087 [Indexed for MEDLINE]<br />
<br />
[2]. J Diet Suppl. 2021;18(3):261-277. doi: 10.1080/19390211.2020.1758274. Epub 2020 Apr 30.<br />
Identification of Erectile Dysfunction Drugs in Dietary Supplements by Liquid Chromatography Ion Trap Mass Spectrometry.<br />
Li C(1), Xu D(1), Moezzi B(1).<br />
Author information: (1)Food and Drug Laboratory Branch, California Department of Public Health, Richmond, CA, USA.<br />
With the rise in consumption of dietary supplements for various ailments such as erectile dysfunction (ED), there is concern that these supplements may contain illegally added phosphodiesterase type 5 (PDE-5) inhibitor and its analogs. HPLC or LC is a general separation method, and MS is a detection technique, together LC/MS/MS technology provides the mass spectral confirmation in identifying sildenafil, vardenafil, tadalafil and their analogs. In our present study, a sample extraction technique with 1:1 acetonitrile: water solvents and sonication was used for screening, then identification was performed using an LC coupled with Velos Pro linear ion trap mass spectrometry. This was a simple and reliable method for a variety of matrices of dietary supplements and pharmaceutical formulations in tablet, capsule or liquid form. The run time is only 6.5 min, allowing for a quick screening and identification of all of analytes of ED drugs using full scan and data-dependent scan MS/MS, except for tadalafil and aminotadalafil (MS/MS/MS). To conclude this study, Sildenafil, tadalafil, vardenafil, and other 16 analogs in dietary supplements could be quickly screened and identified by HPLC coupled with ion trap MS using data dependent scanning function. The main method using the short column is very rapid, and saves a lot of running time and solvents, and the identification is further confirmed by MS/MS information. The current study develops and validates a quick and reliable method to screen for ED drugs.<br />
DOI: 10.1080/19390211.2020.1758274 PMID: 32351143<br />
<br />
[3]. Shokuhin Eiseigaku Zasshi. 2013;54(3):232-6. doi: 10.3358/shokueishi.54.232.<br />
Simultaneous identification of hydroxythiohomosildenafil, aminotadalafil, thiosildenafil, dimethylsildenafil, and thiodimethylsildenafil in dietary supplements using high-performance liquid chromatography-mass spectrometry.<br />
Tagami T(1), Takeda A, Asada A, Aoyama A, Doi T, Kajimura K, Sawabe Y.<br />
Author information: (1)Osaka Prefectural Institute of Public Health, 1-3-69 Nakamichi, Higashinari-ku, Osaka 537-0025, Japan. [email protected]<br />
We developed a method for the separation and identification of illegal adulterants (hydroxythiohomosildenafil, aminotadalafil, thiosildenafil, dimethylsildenafil, and thiodimethylsildenafil) from dietary supplements using high-performance liquid chromatography-mass spectrometry. The separation was achieved on a C18 column: the mobile phase consisted of 5 mM ammonium formate (pH 6.3)-acetonitrile (75 : 25, v/v) and acetonitrile, with gradient elution at a flow rate of 0.2 mL/min. The proposed method could also be used to separate vardenafil, homosildenafil, and dimethylsildenafil, all of which have the same molecular weight. Furthermore, the proposed method could simultaneously separate hydroxythiohomosildenafil, aminotadalafil, thiosildenafil, dimethylsildenafil, thiodimethylsildenafil, vardenafil, and homosildenafil. Thus, this method may be useful to identify medicinal ingredients for erectile dysfunction and their analogs and to control the quality of dietary supplements.<br />
DOI: 10.3358/shokueishi.54.232 PMID: 23863369 [Indexed for MEDLINE]<br />
<br />
[4]. J Sex Med. 2015 Jan;12(1):152-7. doi: 10.1111/jsm.12759. Epub 2014 Nov 17.<br />
Detection of a tadalafil analogue as an adulterant in a dietary supplement for erectile dysfunction.<br />
Ulloa J(1), Sambrotta L, Redko F, Mazza ON, Garrido G, Becher EF, Muschietti L.<br />
Author information: (1)Cátedra de Farmacognosia, Facultad de Farmacia y Bioquímica (UBA), IQUIMEFA (UBA-CONICET), Buenos Aires, Argentina.<br />
Comment in J Urol. 2016 Aug;196(2):514-5.<br />
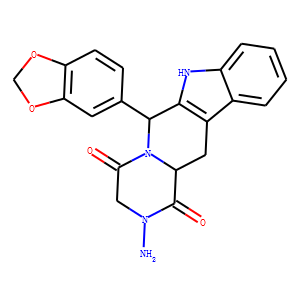
INTRODUCTION: Several cases of adulteration of dietary supplements with tadalafil, sildenafil, and vardenafil, or their unapproved analogues have been reported worldwide. Mainly, the presence of the latter represents a serious health risk to consumers as their efficacy and toxic effects have not been assessed and may result in unpredictable adverse effects. AIM: To investigate the suspected adulteration with synthetic phosphodiesterase type 5 (PDE-5) inhibitors in a dietary supplement marketed in Argentina for the treatment of erectile dysfunction (ED). METHODS: The content of the capsules of the dietary supplement (sample A) was analyzed by thin layer chromatography (TLC) and high-performance liquid chromatography (HPLC) diode-array detection. From the organic extract of sample A, a major compound was purified by column chromatography (CC). The isolated compound was identified by proton nuclear magnetic resonance (1H NMR) and carbon NMR (13C NMR), heteronuclear single quantum coherence, distortionless enhancement by polarization transfer (DEPT 135), electrospray ionization mass spectrometry, and ultraviolet, and infrared (Fourier transform infrared spectroscopy) spectroscopy. MAIN OUTCOME MEASURE: Proof of adulteration of herbal products with synthetic PDE-5 inhibitors. RESULTS: By TLC and HPLC analysis, a major compound was detected in sample A organic extract. The purification of this extract by CC led to the isolation of a pure compound which was identified according to its spectral data as (6R,12aR)-2-amino-6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydropyrazino [1',2':1,6] pyrido [3,4-b] indole-1,4-dione or aminotadalafil. CONCLUSIONS: An unapproved PDE-5 inhibitor analogue, which was identified as aminotadalafil, has been detected in a dietary supplement. This study represents the first report in Latin America and one of the few independent studies of an adulteration with an unapproved PDE-5 inhibitor of an herbal product for ED treatment.<br />
DOI: 10.1111/jsm.12759 PMID: 25402198 [Indexed for MEDLINE]<br />
<br />
[5]. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2013;30(4):621-6. doi: 10.1080/19440049.2013.766766. Epub 2013 Feb 18.<br />
Identification of a new tadalafil analogue found in a dietary supplement.<br />
Lee ES(1), Kim JW, Lee JH, Han KM, Cho S, Hwang I, Han SY, Chae K, Kim J.<br />
Author information: (1)National Institute of Food and Drug Safety Evaluation, Korea Food & Drug Administration, Osong Health Technology Administration Complex, 187 Osongsaengmyeong2-ro, Yeonje-ri, Osongeup, Cheongwon-gun, Chungcheongbuk-do 363-700, Korea.<br />
A new tadalafil analogue, acetaminotadalafil, was detected by HPLC in a bulk powder that is being used as an ingredient formanufacturing dietary supplements. The analogue was isolated by semi-preparative HPLC. A chemical structure of the new compound was elucidated by HPLC, LC-quadrupole time-of-flight mass spectrometry (LC-Q-TOF/MS), nuclear magnetic resonance (NMR), infrared (IR) and circular dichroism (CD) spectroscopy. The compound was identified as an acetylatedcompound of aminotadalafil. The structure of the previous unknown compound was confirmed as (6R,12aR)-2-acetamino-6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-pyrazino[1',2':1,6]pyrido[3,4-b]indole-1,4-dione and named as acetaminotadalafil.<br />
DOI: 10.1080/19440049.2013.766766 PMID: 23419124 [Indexed for MEDLINE]
|