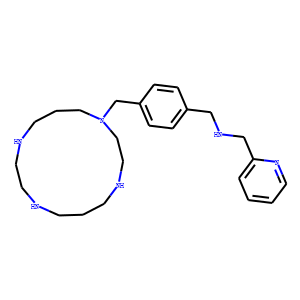
<p style=/line-height:25px/>AMD 3465(GENZ-644494) is a potent, selective CXCR4 antagonist; exhibits 8-fold higher affinity than AMD 3100; inhibits SDF-1α-ligand binding (Ki = 41.7 nM).<br>IC50 value:<br>Target: CXCR4<br>in vitro: AMD3465 is a novel, nonpeptide CXCR4 antagonist and a potent inhibitor of HIV cell entry in that one of the four-nitrogen cyclam rings of the symmetrical, prototype bicyclam antagonist AMD3100 has been replaced by a two-nitrogen N-pyridinylmethylene moiety [1]. AMD3465 is an antagonist of SDF-1 ligand binding (K(i) of 41.7+/-1.2nM), and inhibits SDF-1 mediated signaling as shown by inhibition of GTP binding, calcium flux, and inhibition of chemotaxis. AMD3465 is selective for CXCR4 and does not inhibit chemokine-stimulated calcium flux in cells expressing CXCR3, CCR1, CCR2b, CCR4, CCR5 or CCR7, nor does it inhibit binding of LTB(4) to its receptor, BLT1 [2].<br>in vivo: The pharmacokinetics of AMD3465 was investigated in mice and dogs. Absorption was rapid following subcutaneous administration. AMD3465 was cleared from dog plasma in a biphasic manner with a terminal half-life of 1.56-4.63h. Comparison of exposure to the intravenous and subcutaneous doses indicated 100% bioavailability following subcutaneous administration. AMD3465 caused leukocytosis when administered subcutaneously in mice and dogs, with peak mobilization occurring between 0.5 and 1.5h following subcutaneous dosing in mice and with maximum peak plasma concentration of compound preceding peak mobilization in dogs, indicating that AMD3465 has the potential to mobilize hematopoietic stem cells [2].</p>