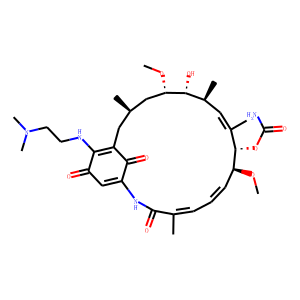
| Synonyms | 17-DMAG ; KOS-1022;[(3R,5S,6R,7S,8E,10S,11S,12Z,14E)-21-[2-(dimethylamino)ethylamino]-6-hydroxy-5,11-dimethoxy-3,7,9,15-tetramethyl-16,20,22-trioxo-17-azabicyclo[16.3.1]docosa-1(21),8,12,14,18-pentaen-10-yl] carbamate
|
| InChI | InChI=1S/C32H48N4O8/c1-18-14-22-27(34-12-13-36(5)6)24(37)17-23(29(22)39)35-31(40)19(2)10-9-11-25(42-7)30(44-32(33)41)21(4)16-20(3)28(38)26(15-18)43-8/h9-11,16-18,20,25-26,28,30,34,38H,12-15H2,1-8H3,(H2,33,41)(H,35,40)/b11-9-,19-10+,21-16+/t18-,20+,25 |
| Reference | 1. Cancer Biol Ther. 2015;16(6):949-57. doi: 10.1080/15384047.2015.1040964. Epub 2015 Apr 28.<br />
The HSP90 inhibitor alvespimycin enhances the potency of telomerase inhibition by imetelstat in human osteosarcoma.<br />
Hu Y(1), Bobb D, He J, Hill DA, Dome JS.<br />
Author information:<br />
(1)a Center for Cancer and Immunology Research and the Division of Oncology; Children/'s National Medical Center ; Washington, DC USA.<br />
The unsatisfactory outcomes for osteosarcoma necessitate novel therapeutic strategies. This study evaluated the effect of the telomerase inhibitor imetelstat in pre-clinical models of human osteosarcoma. Because the chaperone molecule HSP90 facilitates the assembly of telomerase protein, the ability of the HSP90 inhibitor alvespimycin to potentiate the effect of the telomerase inhibitor was assessed. The effect of single or combined treatment with imetelstat and alvespimycin on long-term growth was assessed in osteosarcoma cell lines (143B, HOS and MG-63) and xenografts derived from 143B cells. Results indicated that imetelstat as a single agent inhibited telomerase activity, induced telomere shortening, and inhibited growth in all 3 osteosarcoma cell lines, though the bulk cell cultures did not undergo growth arrest. Combined treatment with imetelstat and alvespimycin resulted in diminished telomerase activity and shorter telomeres compared to either agent alone as well as higher levels of γH2AX and cleaved caspase-3, indicative of increased DNA damage and apoptosis. With dual telomerase and HSP90 inhibition, complete growth arrest of bulk cell cultures was achieved. In xenograft models, all 3 treatment groups significantly inhibited tumor growth compared with the placebo-treated control group, with the greatest effect seen in the combined treatment group (imetelstat, p = 0.045, alvespimycin, p = 0.034; combined treatment, p = 0.004). In conclusion, HSP90 inhibition enhanced the effect of telomerase inhibition in pre-clinical models of osteosarcoma. Dual targeting of telomerase and HSP90 warrants further investigation as a therapeutic strategy.<br />
2. Leukemia. 2010 Apr;24(4):699-705. doi: 10.1038/leu.2009.292. Epub 2010 Jan 28.<br />
Phase I study of the heat shock protein 90 inhibitor alvespimycin (KOS-1022, 17-DMAG) administered intravenously twice weekly to patients with acute myeloid leukemia.<br />
Lancet JE(1), Gojo I, Burton M, Quinn M, Tighe SM, Kersey K, Zhong Z, Albitar MX, Bhalla K, Hannah AL, Baer MR.<br />
Author information:<br />
(1)H Lee Moffitt Cancer Center and Research Institute, Tampa, FL 33612, USA. [email protected]<br />
Heat shock protein 90 (Hsp90) is a molecular chaperone with many oncogenic client proteins. The small-molecule Hsp90 inhibitor alvespimycin, a geldanamycin derivative, is being developed for various malignancies. This phase 1 study examined the maximum-tolerated dose (MTD), safety and pharmacokinetic/pharmacodynamic profiles of alvespimycin in patients with advanced acute myeloid leukemia (AML). Patients with advanced AML received escalating doses of intravenous alvespimycin (8-32 mg/m(2)), twice weekly, for 2 of 3 weeks. Dose-limiting toxicities (DLTs) were assessed during cycle 1. A total of 24 enrolled patients were evaluable for toxicity. Alvespimycin was well tolerated; the MTD was 24 mg/m(2) twice weekly. Common toxicities included neutropenic fever, fatigue, nausea and diarrhea. Cardiac DLTs occurred at 32 mg/m(2) (elevated troponin and myocardial infarction). Pharmacokinetics revealed linear increases in C(max) and area under the curve (AUC) from 8 to 32 mg/m(2) and minor accumulation upon repeated doses. Pharmacodynamic analyses on day 15 revealed increased apoptosis and Hsp70 levels when compared with baseline within marrow blasts. Antileukemia activity occurred in 3 of 17 evaluable patients (complete remission with incomplete blood count recovery). The twice-weekly administered alvespimycin was well tolerated in patients with advanced AML, showing linear pharmacokinetics, target inhibition and signs of clinical activity. We determined a recommended phase 2 dose of 24 mg/m(2).<br />
3. Pediatr Blood Cancer. 2008 Jul;51(1):34-41. doi: 10.1002/pbc.21508.<br />
Stage 1 testing and pharmacodynamic evaluation of the HSP90 inhibitor alvespimycin (17-DMAG, KOS-1022) by the pediatric preclinical testing program.<br />
Smith MA(1), Morton CL, Phelps DA, Kolb EA, Lock R, Carol H, Reynolds CP, Maris JM, Keir ST, Wu J, Houghton PJ.<br />
Author information:<br />
(1)Cancer Therapy Evaluation Program, NCI, Bethesda, Maryland.<br />
BACKGROUND: Alvespimycin (17-DMAG, KOS-1022), a potent small-molecule inhibitor of the protein chaperone Hsp90, is being developed as an anticancer agent because of the multiple Hsp90 client proteins involved in cancer cell growth and survival.<br />
PROCEDURES: Alvespimycin was tested against the in vitro panel of the Pediatric Preclinical Testing Program (PPTP) at concentrations from 1 nM to 10 microM and was tested against the PPTP/'s in vivo tumor panels by intraperitoneal administration using a 50 mg/kg BID twice weekly x 6 weeks dose and schedule. Hsp70 induction in tumor and liver tissue was used as a pharmacodynamic measure of Hsp90 inhibition and stress response induction.<br />
RESULTS: Alvespimycin had a median IC(50) of 68 nM against the PPTP/'s in vitro panel, with a trend for lower IC(50) values for the rhabdomyosarcoma panel (median IC(50) 32 nM) and for higher IC(50) values for the neuroblastoma panel (median IC(50) 380 nM). Using the time to event activity measure, alvespimycin had intermediate or high activity against 4 of 28 evaluable solid tumor xenografts, including 3 of 4 alveolar rhabdomyosarcoma xenografts (one with a partial response). Hsp70 induction was observed in tumor tissue from both responding and non-responding xenografts.<br />
CONCLUSIONS: Alvespimycin demonstrated little in vivo antitumor activity against most of the PPTP/'s preclinical models. The greatest drug effect was observed for the alveolar rhabdomyosarcoma xenografts in the rhabdomyosarcoma panel. Hsp70 induction was observed in responding and non-responding xenografts, suggesting that tumor-specific events subsequent to HSP90 inhibition are primary determinants of antitumor activity.<br />
|