| Catalog Number | R033143 |
| CAS Number | 113665-89-7 |
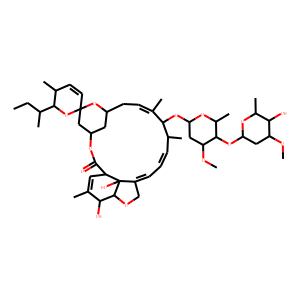
| Molecular Formula | C48H72O14
|
| Purity | 95% |
| Storage | -20°C |
| IUPAC Name | (2R,3S,4'S,6S,8'R,10'E,13'S,14'E,16'E,20'R,21'R,24'S)-2-butan-2-yl-21',24'-dihydroxy-12'-[5-(5-hydroxy-4-methoxy-6-methyloxan-2-yl)oxy-4-methoxy-6-methyloxan-2-yl]oxy-3,11',13',22'-tetramethylspiro[2,3-dihydropyran-6,6'-3,7,19-trioxatetracyclo[15.6.1.14,8.020,24]pentacosa-10,14,16,22-tetraene]-2'-one |
| InChI | InChI=1S/C48H72O14/c1-11-25(2)43-28(5)17-18-47(62-43)23-34-20-33(61-47)16-15-27(4)42(26(3)13-12-14-32-24-55-45-40(49)29(6)19-35(46(51)58-34)48(32,45)52)59-39-22-37(54-10)44(31(8)57-39)60-38-21-36(53-9)41(50)30(7)56-38/h12-15,17-19,25-26,28,30-31,33-45,49-50,52H,11,16,20-24H2,1-10H3/b13-12+,27-15+,32-14+/t25?,26-,28-,30?,31?,33+,34-,35?,36?,37?,38?,39?,40+,41?,42?,43+,44?,45+,47+,48+/m0/s1 |
| InChIKey | RRZXIRBKKLTSOM-NJRQVPCTSA-N |
| SMILES | CCC(C)C1C(C=CC2(O1)CC3CC(O2)CC=C(C(C(C=CC=C4COC5C4(C(C=C(C5O)C)C(=O)O3)O)C)OC6CC(C(C(O6)C)OC7CC(C(C(O7)C)O)OC)OC)C)C |
| Reference | <span style="color:#000000;"><span style="font-family:arial,helvetica,sans-serif;"><span style="font-size:12px;">1.<span style="font-variant-ligatures: normal; orphans: 2; widows: 2;">Lofgren, C. S., and D. F. Williams. "Avermectin B1a: highly potent inhibitor of reproduction by queens of the red imported fire ant (Hymenoptera: Formicidae)." </span><i style="font-family: Arial, sans-serif; font-size: 13px; font-variant-ligatures: normal; orphans: 2; widows: 2;">Journal of Economic Entomology</i><span style="font-variant-ligatures: normal; orphans: 2; widows: 2;"> 75.5 (1982): 798-803.<br />
2.</span><span style="font-variant-ligatures: normal; orphans: 2; widows: 2;">Kass, I. S., et al. "Avermectin B1a, a paralyzing anthelmintic that affects interneurons and inhibitory motoneurons in Ascaris." </span><i style="font-family: Arial, sans-serif; font-size: 13px; font-variant-ligatures: normal; orphans: 2; widows: 2;">Proceedings of the National Academy of Sciences</i><span style="font-variant-ligatures: normal; orphans: 2; widows: 2;"> 77.10 (1980): 6211-6215.<br />
3.</span><span style="font-variant-ligatures: normal; orphans: 2; widows: 2;">Fritz, Lawrence C., Ching C. Wang, and Alfredo Gorio. "Avermectin B1a irreversibly blocks postsynaptic potentials at the lobster neuromuscular junction by reducing muscle membrane resistance." </span><i style="font-family: Arial, sans-serif; font-size: 13px; font-variant-ligatures: normal; orphans: 2; widows: 2;">Proceedings of the National Academy of Sciences</i><span style="font-variant-ligatures: normal; orphans: 2; widows: 2;"> 76.4 (1979): 2062-2066.<br />
4.</span><span style="font-variant-ligatures: normal; orphans: 2; widows: 2;">Maynard, Michael S., et al. "Fate of the 8, 9-Z isomer of avermectin B1a in rats." </span><i style="font-family: Arial, sans-serif; font-size: 13px; font-variant-ligatures: normal; orphans: 2; widows: 2;">Journal of Agricultural and Food Chemistry</i><span style="font-variant-ligatures: normal; orphans: 2; widows: 2;"> 37.6 (1989): 1487-1491.<br />
5.</span><span style="font-variant-ligatures: normal; orphans: 2; widows: 2;">Maynard, Michael S., and Heather D. Maynard. "HPLC assay for avermectin B1a and its two photoisomers using a photo diode array detector." </span><i style="font-family: Arial, sans-serif; font-size: 13px; font-variant-ligatures: normal; orphans: 2; widows: 2;">Bulletin of environmental contamination and toxicology</i><span style="font-variant-ligatures: normal; orphans: 2; widows: 2;"> 43.4 (1989): 499-504.<br />
6.</span><span style="font-variant-ligatures: normal; orphans: 2; widows: 2;">Crouch, Louis S., et al. "Photodegradation of avermectin B1a thin films on glass." </span><i style="font-family: Arial, sans-serif; font-size: 13px; font-variant-ligatures: normal; orphans: 2; widows: 2;">Journal of agricultural and food chemistry</i><span style="font-variant-ligatures: normal; orphans: 2; widows: 2;"> 39.7 (1991): 1310-1319.</span></span></span></span>
|