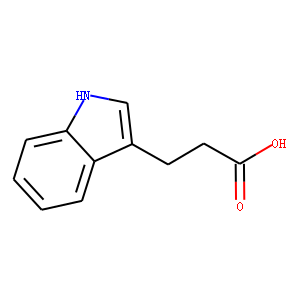
| Reference | </br>1: Köse M, Ritter K, Thiemke K, Gillard M, Kostenis E, Müller CE. Development of [(3)H]2-Carboxy-4,6-dichloro-1H-indole-3-propionic Acid ([(3)H]PSB-12150): A Useful Tool for Studying GPR17. ACS Med Chem Lett. 2014 Jan 16;5(4):326-30. doi: 10.1021/ml400399f. PubMed PMID: 24900835; PubMed Central PMCID: PMC4027623.</br>2: Hwang IK, Yoo KY, Li H, Park OK, Lee CH, Choi JH, Jeong YG, Lee YL, Kim YM, Kwon YG, Won MH. Indole-3-propionic acid attenuates neuronal damage and oxidative stress in the ischemic hippocampus. J Neurosci Res. 2009 Jul;87(9):2126-37. doi: 10.1002/jnr.22030. PubMed PMID: 19235887.</br>3: Karbownik M, Stasiak M, Zygmunt A, Zasada K, Lewiński A. Protective effects of melatonin and indole-3-propionic acid against lipid peroxidation, caused by potassium bromate in the rat kidney. Cell Biochem Funct. 2006 Nov-Dec;24(6):483-9. PubMed PMID: 16397908.</br>4: Karbownik M, Stasiak M, Zasada K, Zygmunt A, Lewinski A. Comparison of potential protective effects of melatonin, indole-3-propionic acid, and propylthiouracil against lipid peroxidation caused by potassium bromate in the thyroid gland. J Cell Biochem. 2005 May 1;95(1):131-8. PubMed PMID: 15723291.</br>5: Ostrowski M, Hetmann A, Jakubowska A. Indole-3-acetic acid UDP-glucosyltransferase from immature seeds of pea is involved in modification of glycoproteins. Phytochemistry. 2015 Sep;117:25-33. doi: 10.1016/j.phytochem.2015.05.023. PubMed PMID: 26057226.</br>6: Bendheim PE, Poeggeler B, Neria E, Ziv V, Pappolla MA, Chain DG. Development of indole-3-propionic acid (OXIGON) for Alzheimer/’s disease. J Mol Neurosci. 2002 Aug-Oct;19(1-2):213-7. Review. PubMed PMID: 12212784.</br>7: Karbownik M, Garcia JJ, Lewiński A, Reiter RJ. Carcinogen-induced, free radical-mediated reduction in microsomal membrane fluidity: reversal by indole-3-propionic acid. J Bioenerg Biomembr. 2001 Feb;33(1):73-8. PubMed PMID: 11460928.</br>8: Karbownik M, Reiter RJ, Garcia JJ, Cabrera J, Burkhardt S, Osuna C, Lewiński A. Indole-3-propionic acid, a melatonin-related molecule, protects hepatic microsomal membranes from iron-induced oxidative damage: relevance to cancer reduction. J Cell Biochem. 2001;81(3):507-13. PubMed PMID: 11255233.</br>9: Bajguz A, Piotrowska-Niczyporuk A. Synergistic effect of auxins and brassinosteroids on the growth and regulation of metabolite content in the green alga Chlorella vulgaris (Trebouxiophyceae). Plant Physiol Biochem. 2013 Oct;71:290-7. doi: 10.1016/j.plaphy.2013.08.003. PubMed PMID: 23994360.</br>10: Chyan YJ, Poeggeler B, Omar RA, Chain DG, Frangione B, Ghiso J, Pappolla MA. Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid. J Biol Chem. 1999 Jul 30;274(31):21937-42. PubMed PMID: 10419516.</br>11: Tolan D, Gandin V, Morrison L, El-Nahas A, Marzano C, Montagner D, Erxleben A. Oxidative Stress Induced by Pt(IV) Pro-drugs Based on the Cisplatin Scaffold and Indole Carboxylic Acids in Axial Position. Sci Rep. 2016 Jul 11;6:29367. doi: 10.1038/srep29367. PubMed PMID: 27404565; PubMed Central PMCID: PMC4941645.</br>12: Bosco R, Caser M, Vanara F, Scariot V. Development of a rapid LC-DAD/FLD method for the simultaneous determination of auxins and abscisic acid in plant extracts. J Agric Food Chem. 2013 Nov 20;61(46):10940-7. doi: 10.1021/jf4034305. PubMed PMID: 24134056.</br>13: Edwards N, Anderson CM, Gatfield KM, Jevons MP, Ganapathy V, Thwaites DT. Amino acid derivatives are substrates or non-transported inhibitors of the amino acid transporter PAT2 (slc36a2). Biochim Biophys Acta. 2011 Jan;1808(1):260-70. doi: 10.1016/j.bbamem.2010.07.032. PubMed PMID: 20691150; PubMed Central PMCID: PMC3000476.</br>14: Wang L, Wang M, Yan H, Yuan Y, Tian J. A new graphene oxide/polypyrrole foam material with pipette-tip solid-phase extraction for determination of three auxins in papaya juice. J Chromatogr A. 2014 Nov 14;1368:37-43. doi: 10.1016/j.chroma.2014.09.059. PubMed PMID: 25441342.</br>15: Mariani ME, Madoery RR, Fidelio GD. Auxins action on Glycine max secretory phospholipase A2 is mediated by the interfacial properties imposed by the phytohormones. Chem Phys Lipids. 2015 Jul;189:1-6. doi: 10.1016/j.chemphyslip.2015.05.003. PubMed PMID: 25987194.</br>16: Castillo G, Torrecillas A, Nogueiras C, Michelena G, Sánchez-Bravo J, Acosta M. Simultaneous quantification of phytohormones in fermentation extracts of Botryodiplodia theobromae by liquid chromatography-electrospray tandem mass spectrometry. World J Microbiol Biotechnol. 2014 Jul;30(7):1937-46. doi: 10.1007/s11274-014-1612-5. PubMed PMID: 24510403.</br>17: Tomasić A, Bertosa B, Tomić S, Soskić M, Magnus V. Binding behavior of amino acid conjugates of indole-3-acetic acid to immobilized human serum albumin. J Chromatogr A. 2007 Jun 22;1154(1-2):240-9. PubMed PMID: 17459401.</br>18: Simon S, Kubeš M, Baster P, Robert S, Dobrev PI, Friml J, Petrášek J, Zažímalová E. Defining the selectivity of processes along the auxin response chain: a study using auxin analogues. New Phytol. 2013 Dec;200(4):1034-48. doi: 10.1111/nph.12437. PubMed PMID: 23914741.</br>19: Jin SH, Ma XM, Han P, Wang B, Sun YG, Zhang GZ, Li YJ, Hou BK. UGT74D1 is a novel auxin glycosyltransferase from Arabidopsis thaliana. PLoS One. 2013 Apr 16;8(4):e61705. doi: 10.1371/journal.pone.0061705. Erratum in: PLoS One. 2013;8(8). doi:10.1371/annotation/457d7567-fc12-421c-9d79-880950ab10e1. PubMed PMID: 23613909; PubMed Central PMCID: PMC3628222.</br>20: Barkawi LS, Tam YY, Tillman JA, Pederson B, Calio J, Al-Amier H, Emerick M, Normanly J, Cohen JD. A high-throughput method for the quantitative analysis of indole-3-acetic acid and other auxins from plant tissue. Anal Biochem. 2008 Jan 15;372(2):177-88. PubMed PMID: 17889819.</br>
|