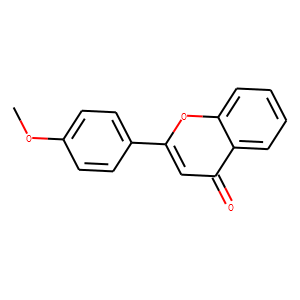
| Reference | [1]. Br J Pharmacol. 2013 Jul;169(6):1263-78. doi: 10.1111/bph.12201.<br />
Identification through high-throughput screening of 4'-methoxyflavone and 3',4'-dimethoxyflavone as novel neuroprotective inhibitors of parthanatos.<br />
Fatokun AA(1), Liu JO, Dawson VL, Dawson TM.<br />
Author information: (1)Neuroregeneration and Stem Cell Programs, Institute for Cell Engineering, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA. [email protected]<br />
BACKGROUND AND PURPOSE: The current lack of disease-modifying therapeutics to manage neurological and neurodegenerative conditions justifies the development of more efficacious agents. One distinct pathway leading to neuronal death in these conditions and which represents a very promising and attractive therapeutic target is parthanatos, involving overactivation of PARP-1. We therefore sought to identify small molecules that could be neuroprotective by targeting the pathway. EXPERIMENTAL APPROACH: Using HeLa cells, we developed and optimized an assay for high-throughput screening of about 5120 small molecules. Structure-activity relationship (SAR) study was carried out in HeLa and SH-SY5Y cells for molecules related to the initial active compound. The neuroprotective ability of each active compound was tested in cortical neuronal cultures. KEY RESULTS: 4'-Methoxyflavone (4MF) showed activity by preventing the decrease in cell viability of HeLa and SH-SY5Y cells caused by the DNA-alkylating agent, N-methyl-N'-nitro-N-nitrosoguanidine (MNNG), which induces parthanatos. A similar compound from the SAR study, 3',4'-dimethoxyflavone (DMF), also showed significant activity. Both compounds reduced the synthesis and accumulation of poly (ADP-ribose) polymer and protected cortical neurones against cell death induced by NMDA. CONCLUSIONS AND IMPLICATIONS: Our data reveal additional neuroprotective members of the flavone class of flavonoids and show that methoxylation of the parent flavone structure at position 4' confers parthanatos-inhibiting activity while additional methoxylation at position 3', reported by others to improve metabolic stability, does not destroy the activity. These molecules may therefore serve as leads for the development of novel neurotherapeutics for the management of neurological and neurodegenerative conditions.<br />
DOI: 10.1111/bph.12201 PMCID: PMC3831707 PMID: 23550801 [Indexed for MEDLINE]<br />
<br />
[2]. Int J Mol Med. 2014 Feb;33(2):317-24. doi: 10.3892/ijmm.2013.1571. Epub 2013 Nov 27.<br />
Acacetin (5,7-dihydroxy-4'-methoxyflavone) exhibits in vitro and in vivo anticancer activity through the suppression of NF-κB/Akt signaling in prostate cancer cells.<br />
Kim HR(1), Park CG(2), Jung JY(1).<br />
Author information: (1)Department of Companion and Laboratory Animal Science, Kongju National University, Yesan 340-702, Republic of Korea. (2)Department of Rural Construction Engineering, Kongju National University, Yesan 340-702, Republic of Korea.<br />
Acacetin (5,7-dihydroxy-4'-methoxyflavone) is a flavonoid compound with antimutagenic, antiplasmodial, antiperoxidant, anti-inflammatory and anticancer effects. However, the molecular targets and pathways underlying the anticancer effects of acacetin are yet to be elucidated. In this study, we investigated whether acacetin induces apoptosis in the human prostate cancer cell line, DU145. The results of 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assays revealed that cell viability decreased in a dose- and time-dependent manner in response to acacetin. 4',6-Diamidino-2-phenylindole (DAPI) staining revealed that chromatin condensation significantly increased in a dose-dependent manner. Flow cytometric analysis indicated that acacetin suppressed the viability of DU145 cells by inducing apoptosis. Western blot anlaysis of various markers of signaling pathways revealed that acacetin targets the Akt and nuclear factor (NF)-κB signaling pathways by inhibiting the phosphorylation of IκBα and NF-κB in a dose-dependent manner. Consistent with its ability to induce apoptosis, the acacetin-mediated inhibition of the pro-survival pathway, Akt, and of the NF-κB pathway was accompanied by a marked reduction in the levels of the NF-κB‑regulated anti-apoptotic proteins, Bcl-2 and X-linked inhibitor of apoptosis protein (XIAP), as well as of the proliferative protein, cyclooxygenase (COX)-2. We further evaluated the effects of acacetin on prostate cancer using mice subcutaneously injected with DU145 prostate cancer cells. The acacetin-treated nude mice bearing DU145 tumor xenografts exhibited significantly reduced tumor size and weight, due to the effects of acacetin on cancer cell apoptosis, as determined by terminal deoxyribonucleotide transferase-mediated dUTP nick end-labeling (TUNEL) assay. Our findings suggest that acacetin exerts antitumor effects by targeting the Akt/NF-κB signaling pathway. Rurther investigations on this flavonoid are warranted to evaluate its potential use in the prevention and therapy of prostate cancer.<br />
DOI: 10.3892/ijmm.2013.1571 PMID: 24285354 [Indexed for MEDLINE]<br />
<br />
[3]. Biochim Biophys Acta. 2013 Jun;1833(6):1316-28. doi: 10.1016/j.bbamcr.2013.02.016. Epub 2013 Feb 26.<br />
p70S6 kinase is a target of the novel proteasome inhibitor 3,3'-diamino-4'-methoxyflavone during apoptosis in human myeloid tumor cells.<br />
Piedfer M(1), Bouchet S, Tang R, Billard C, Dauzonne D, Bauvois B.<br />
Author information: (1)Université Pierre et Marie Curie, Université Paris-Descartes, Centre de Recherche des Cordeliers, Paris, France.<br />
Acute myeloid leukemia (AML) is a deadly disease characterized by the clonal expansion and accumulation of hematopoietic stem cells arrested at various stages of development. Clinical research efforts are currently focusing on targeted therapies that induce apoptosis in AML cells. Herein, the effects and mechanisms of the novel flavone 3,3'-diamino-4'-methoxyflavone (DD1) on AML cell dysfunction were investigated in AML cells (monoblast U937, myelomonocyte OCI-AML3, promyelocyte NB4, myeloblast HL-60) and blood samples from patients with AML. The administration of DD1 inhibited proliferation and induced death of AML cell lines and reduced the clonogenic activity of AML, but not normal, blood cells. The flavone's apoptotic action in U937 cells was associated with recruitment of mitochondria, Bax activation, Bad dephosphorylation (at Ser(136)), activation of caspases -8, -9, and -3 and cleavage of the caspase substrate PARP-1. DD1 induced a marked decrease in (i) Thr(389)-phosphorylation and (ii) protein levels of the caspase-3 substrate P70 ribosomal S6 kinase (P70S6K, known for its ability to phosphorylate Bad). Caspase-dependent apoptosis and P70S6K degradation were simultaneously prevented by the caspase inhibitors. Importantly, DD1 was shown to directly inhibit the proteasome's chymotrypsin-like activity in U937 cells. Apoptotic activity of the proteasome inhibitor bortezomib was also related to Bax activation and P70S6K downregulation. Accordingly, DD1 failed to induce P70S6K cleavage, Bax stimulation and apoptosis in K562 cells resistant to bortezomib. These results indicate that DD1 has the potential to eradicate AML cells and support a critical role for Bax and P70S6K in DD1-mediated proteasome inhibition and apoptosis of leukemia cells.<br />
DOI: 10.1016/j.bbamcr.2013.02.016 PMID: 23481040 [Indexed for MEDLINE]<br />
<br />
[4]. Int J Pept Protein Res. 1996 May;47(5):323-32. doi: 10.1111/j.1399-3011.1996.tb01080.x.<br />
Interaction of 5,7-dihydroxy-4'-methoxyflavone with a multisubunit protein, carmin: thermodynamics and kinetics of interaction.<br />
Rao KS(1), Suryaprakash P, Prakash V.<br />
Author information: (1)Department of Protein Chemistry and Technology, Central Food Technological Research Institute, Mysore, India.<br />
Acacetin (5,7-dihydroxy-4'-methoxy flavone) is a flavone intrinsically present in the seeds of Carthamus tinctorius. Carmin is a multimeric, high molecular weight protein from the seeds of Carthamus tinctorius. The association constant of interaction of acacetin and carmin is maximum at 37.2 degrees C with a value of (3.96 +/- 0.61) x 10(4) M-1 as measured by fluorescence quenching. Acacetin has at least two binding sites on carmin. The interaction follows pseudo-first-order kinetics with a reaction rate constant of 3.4 +/- 0.4 s-1. The titration calorimetric data suggest that binding sites for acacetin and its structural analogue, biochanin A, are conserved. The interaction does not affect the association-dissociation equilibrium of the protein. Also, the binding does not induce any significant conformational changes in the protein as monitored by circular dichroic spectra. Biochanin A (5, 7-dihydroxy-4'-methoxyisoflavone), a structural analogue, interacts with carmin with an association constant of (9.33 +/- 1.44) x 10(4) M-1 at 36.9 degrees C. This indicates that the stereochemistry of the ligand plays an important role in the binding process of flavone to protein. Interaction studies of chemically modified lysyl and tryptophanyl groups separately, and lysyl and tryptophanyl groups sequentially, in the protein carmin with the ligands reveal the involvement of tryptophanyl residues in the binding process and show that it is predominantly an entropically driven hydrophobic interaction.<br />
DOI: 10.1111/j.1399-3011.1996.tb01080.x PMID: 8791154 [Indexed for MEDLINE]<br />
<br />
[5]. Phytochemistry. 2002 Aug;60(7):727-31. doi: 10.1016/s0031-9422(02)00192-9.<br />
Scutellarein 4'-methyl ether glycosides as taxonomic markers in Teucridium and Tripora (Lamiaceae, Ajugoideae).<br />
Grayer RJ(1), Veitch NC, Kite GC, Paton AJ, Garnock-Jones PJ.<br />
Author information: (1)Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, TW9 3DS, Surrey, UK. [email protected]<br />
The flavonoid profiles of two monotypic genera, Teucridium and Tripora, have been studied by analytical methods. These genera were formerly placed in the Verbenaceae, but are now classified in the Lamiaceae, subfamily Ajugoideae. The major flavonoids of both genera were identified as glycosides of scutellarein 4'-methyl ether (5,6,7-trihydroxy-4'methoxyflavone) and acacetin (5,7-dihydroxy-4'-methoxyflavone). The new flavone glycoside, scutellarein 4'-methyl ether 7-O-rutinoside, was isolated from Teucridium parvifolium and the rare scutellarein 4'-methyl ether 7-O-glucuronide from Tripora divaricata. The latter compound has only been reported previously in the related genus Clerodendron. The potential of these flavonoids as taxonomic markers for the tribe Ajugoideae is discussed.<br />
DOI: 10.1016/s0031-9422(02)00192-9 PMID: 12127590 [Indexed for MEDLINE]
|