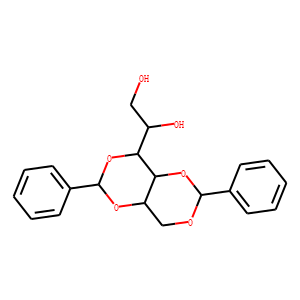
| Reference | <span style="font-family: Arial, sans-serif; font-size: 13px;">1. </span><span style="font-family: Arial, sans-serif; font-size: 13px;">The Journal of Physical Chemistry B. 2001 Mar 22;105(11):2091-8.</span><span style="font-family: Arial, sans-serif; font-size: 13px;">Morphological characteristics of 1, 3: 2, 4-dibenzylidene sorbitol/poly (propylene glycol) organogels.</span><span style="font-family: Arial, sans-serif; font-size: 13px;"> Mercurio DJ, Spontak RJ.</span><br />
<br />
<span style="font-family:arial,helvetica,sans-serif;"><span style="font-size:12px;"><span style="caret-color: rgb(0, 0, 0);">In this work, the morphological characteristics of organogels composed of </span></span></span>(1,3:2,4) DIBENZYLIDENE SORBITOL<span style="font-family:arial,helvetica,sans-serif;"><span style="font-size:12px;"><span style="caret-color: rgb(0, 0, 0);"> (DBS) and poly(propylene glycol) (PPG) are investigated as functions of DBS concentration, PPG molecular weight, and temperature through the use of polarized light microscopy, transmission electron microscopy, X-ray diffractometry, and spectrophotometry. Polarized light microscopy reveals thermally reversible features that become increasingly more pronounced with increasing DBS concentration. Electron microscopy verifies that these features arise due to the presence of a DBS nanofibrillar network, with nanofibrils measuring ca. 10 nm in diameter. Comparison of X-ray diffraction patterns of pure DBS crystals and DBS networks from which PPG is removed by supercritical fluid extraction reveals that the DBS nanofibrils are crystalline, differing slightly from the structure of pure DBS. Spectrophotometry is used to probe the temperature-dependent development of the molecular network in DBS/PPG organogels.<br />
<br />
2.</span></span></span><span style="font-family: Arial, sans-serif; font-size: 13px;">Langmuir. 2014 Dec 2;30(47):14128-42. </span><span style="font-family: Arial, sans-serif; font-size: 13px;">Comparing and correlating solubility parameters governing the self-assembly of molecular gels using 1, 3: 2, 4-dibenzylidene sorbitol as the gelator.</span><span style="font-family: Arial, sans-serif; font-size: 13px;">Lan Y, Corradini MG, Liu X, May TE, Borondics F, Weiss RG, Rogers MA.</span><br />
<br />
<span style="font-size:12px;"><span style="font-family:arial,helvetica,sans-serif;"><span style="caret-color: rgb(0, 0, 0);">Solvent properties play a central role in mediating the aggregation and self-assembly of molecular gelators and their growth into fibers. Numerous attempts have been made to correlate the solubility parameters of solvents and gelation abilities of molecular gelators, but a comprehensive comparison of the most important parameters has yet to appear. Here, the degree to which partition coefficients (log </span><i style="box-sizing: border-box; outline: none; caret-color: rgb(0, 0, 0); color: rgb(0, 0, 0); font-family: Georgia, serif; font-size: 17px;">P</i><span style="caret-color: rgb(0, 0, 0);">), Henry’s law constants (HLC), dipole moments, static relative permittivities (ε</span><span style="box-sizing: border-box; outline: none; line-height: 0; position: relative; vertical-align: baseline; bottom: -0.25em; caret-color: rgb(0, 0, 0);">r</span><span style="caret-color: rgb(0, 0, 0);">), solvatochromic </span><i style="box-sizing: border-box; outline: none; caret-color: rgb(0, 0, 0); color: rgb(0, 0, 0); font-family: Georgia, serif; font-size: 17px;">E</i><span style="box-sizing: border-box; outline: none; line-height: 0; position: relative; vertical-align: baseline; bottom: -0.25em; caret-color: rgb(0, 0, 0);">T</span><span style="caret-color: rgb(0, 0, 0);">(30) parameters, Kamlet–Taft parameters (</span><i style="box-sizing: border-box; outline: none; caret-color: rgb(0, 0, 0); color: rgb(0, 0, 0); font-family: Georgia, serif; font-size: 17px;">β</i><span style="caret-color: rgb(0, 0, 0);">,</span><i style="box-sizing: border-box; outline: none; caret-color: rgb(0, 0, 0); color: rgb(0, 0, 0); font-family: Georgia, serif; font-size: 17px;"> α</i><span style="caret-color: rgb(0, 0, 0);">, and π), Catalan’s solvatochromic parameters (SPP, SB, and SA), Hildebrand solubility parameters (δ</span><span style="box-sizing: border-box; outline: none; line-height: 0; position: relative; vertical-align: baseline; bottom: -0.25em; caret-color: rgb(0, 0, 0);"><i style="box-sizing: border-box; outline: none;">i</i></span><span style="caret-color: rgb(0, 0, 0);">), and Hansen solubility parameters (δ</span><span style="box-sizing: border-box; outline: none; line-height: 0; position: relative; vertical-align: baseline; bottom: -0.25em; caret-color: rgb(0, 0, 0);">p</span><span style="caret-color: rgb(0, 0, 0);">, δ</span><span style="box-sizing: border-box; outline: none; line-height: 0; position: relative; vertical-align: baseline; bottom: -0.25em; caret-color: rgb(0, 0, 0);">d</span><span style="caret-color: rgb(0, 0, 0);">, δ</span><span style="box-sizing: border-box; outline: none; line-height: 0; position: relative; vertical-align: baseline; bottom: -0.25em; caret-color: rgb(0, 0, 0);">h</span><span style="caret-color: rgb(0, 0, 0);">) and the associated Hansen distance (</span><i style="box-sizing: border-box; outline: none; caret-color: rgb(0, 0, 0); color: rgb(0, 0, 0); font-family: Georgia, serif; font-size: 17px;">R</i><span style="box-sizing: border-box; outline: none; line-height: 0; position: relative; vertical-align: baseline; bottom: -0.25em; caret-color: rgb(0, 0, 0);"><i style="box-sizing: border-box; outline: none;">ij</i></span><span style="caret-color: rgb(0, 0, 0);">) of 62 solvents (covering a wide range of properties) can be correlated with the self-assembly and gelation of 1,3:2,4-dibenzylidene sorbitol (DBS) gelation, a classic molecular gelator, is assessed systematically. The approach presented describes the basis for each of the parameters and how it can be applied. As such, it is an instructional blueprint for how to assess the appropriate type of solvent parameter for use with other molecular gelators as well as with molecules forming other types of self-assembled materials. The results also reveal several important insights into the factors favoring the gelation of solvents by DBS. The ability of a solvent to accept or donate a hydrogen bond is much more important than solvent polarity in determining whether mixtures with DBS become solutions, clear gels, or opaque gels. Thermodynamically derived parameters could not be correlated to the physical properties of the molecular gels unless they were dissected into their individual HSPs. The DBS solvent phases tend to cluster in regions of Hansen space and are highly influenced by the hydrogen-bonding HSP, δ</span><span style="box-sizing: border-box; outline: none; line-height: 0; position: relative; vertical-align: baseline; bottom: -0.25em; caret-color: rgb(0, 0, 0);">h</span><span style="caret-color: rgb(0, 0, 0);">. It is also found that the fate of this molecular gelator, unlike that of polymers, is influenced not only by the magnitude of the distance between the HSPs for DBS and the HSPs of the solvent, </span><i style="box-sizing: border-box; outline: none; caret-color: rgb(0, 0, 0); color: rgb(0, 0, 0); font-family: Georgia, serif; font-size: 17px;">R</i><span style="box-sizing: border-box; outline: none; line-height: 0; position: relative; vertical-align: baseline; bottom: -0.25em; caret-color: rgb(0, 0, 0);"><i style="box-sizing: border-box; outline: none;">ij</i></span><span style="caret-color: rgb(0, 0, 0);">, but also by the directionality of </span><i style="box-sizing: border-box; outline: none; caret-color: rgb(0, 0, 0); color: rgb(0, 0, 0); font-family: Georgia, serif; font-size: 17px;">R</i><span style="box-sizing: border-box; outline: none; line-height: 0; position: relative; vertical-align: baseline; bottom: -0.25em; caret-color: rgb(0, 0, 0);"><i style="box-sizing: border-box; outline: none;">ij</i></span><span style="caret-color: rgb(0, 0, 0);">: if the solvent has a larger hydrogen-bonding HSP (indicating stronger H-bonding) than that of the DBS, then clear gels are formed; opaque gels form when the solvent has a lower δ</span><span style="box-sizing: border-box; outline: none; line-height: 0; position: relative; vertical-align: baseline; bottom: -0.25em; caret-color: rgb(0, 0, 0);">h</span><span style="caret-color: rgb(0, 0, 0);"> than does DBS.<br />
<br />
3.</span>Journal of Materials Chemistry. 1995 Jan 1;5(11):1899-903.Gelation of silicone fluids using 1, 3: 2, 4-dibenzylidene sorbitol.Smith JM, Katsoulis DE.<br />
<br />
<span style="caret-color: rgba(0, 0, 0, 0.792157);">A variety of silicone fluids have been found to gel using low concentrations (typically < 4 wt.%) of 1,3:2,4-dibenzylidene sorbitol (DBS). DBS is known to be a chiral gelator for many organic solvents. Gels were formed when small amounts of DBS were introduced into the silicone fluids either by heating to high temperatures or at ambient temperatures with the use of a co-solvent. Optical and electron microscopy of neat silicon–DBS gels (concentration as low as 0.005 wt.%), revealed the formation of two types of fibrous network. One consisted of ribbon-like macrofibres (average width, </span><span class="italic" style="font-style: italic; line-height: 1.875em; caret-color: rgba(0, 0, 0, 0.792157);">ca.</span><span style="caret-color: rgba(0, 0, 0, 0.792157);"> 2–3 µm) present in the opaque region of the gels and the other consisted of dense intertwined microfibres (average width, </span><span class="italic" style="font-style: italic; line-height: 1.875em; caret-color: rgba(0, 0, 0, 0.792157);">ca.</span><span style="caret-color: rgba(0, 0, 0, 0.792157);"> 100 nm) present in the clear portion of the gels. The gels prepared in the presence of a co-solvent consisted only of the microfibrous network. </span><span class="italic" style="font-style: italic; line-height: 1.875em; caret-color: rgba(0, 0, 0, 0.792157);">N</span><span style="caret-color: rgba(0, 0, 0, 0.792157);">-Methylpyrrolidone was found to be a very effective co-solvent for the phenyl-containing siloxanes producing firm clear gels. Dynamic mechanical measurements indicated that the storage modulus (</span><span class="italic" style="font-style: italic; line-height: 1.875em; caret-color: rgba(0, 0, 0, 0.792157);">G</span><span style="caret-color: rgba(0, 0, 0, 0.792157);">′) of these gels increased with increasing DBS content over the range 1–6 wt.% DBS. Comparison with propylene glycol–DBS gels showed the silicone gels to differ in that they comprised mostly an isotropic phase with no detectable crystalline phase present.</span></span></span>
|