| Reference | [1]. Nucleic Acids Res. 2020 Apr 6;48(6):e35. doi: 10.1093/nar/gkaa070.<br />
N 1-Methylpseudouridine substitution enhances the performance of synthetic mRNA switches in cells.<br />
Parr CJC(1), Wada S(1), Kotake K(1), Kameda S(1), Matsuura S(1), Sakashita S(2), Park S(2), Sugiyama H(2), Kuang Y(3), Saito H(1).<br />
Author information: (1)Department of Life Science Frontiers, Center for iPS Cell Research and Application, Kyoto University, 53, Kawaharacho, Sakyo-ku, Kyoto 606-8507, Japan. (2)Department of Chemistry, Graduate School of Science, Kyoto University, Kitashirakawa-Oiwakecho, Sakyo-Ku, Kyoto 606-8502, Japan. (3)Department of Chemical and Biological Engineering, Hong Kong University of Science and Technology, Room 5578, Academic Bldg, Clear Water Bay, Kowloon, Hong Kong.<br />
Synthetic messenger RNA (mRNA) tools often use pseudouridine and 5-methyl cytidine as substitutions for uridine and cytidine to avoid the immune response and cytotoxicity induced by introducing mRNA into cells. However, the influence of base modifications on the functionality of the RNA tools is poorly understood. Here we show that synthetic mRNA switches containing N1-methylpseudouridine (m1Ψ) as a substitution of uridine substantially out-performed all other modified bases studied, exhibiting enhanced microRNA and protein sensitivity, better cell-type separation ability, and comparably low immune stimulation. We found that the observed phenomena stem from the high protein expression from m1Ψ containing mRNA and efficient translational repression in the presence of target microRNAs or proteins. In addition, synthetic gene circuits with m1Ψ significantly improve performance in cells. These findings indicate that synthetic mRNAs with m1Ψ modification have enormous potentials in the research and application of biofunctional RNA tools.<br />
DOI: 10.1093/nar/gkaa070 PMCID: PMC7102939 PMID: 32090264 [Indexed for MEDLINE]<br />
<br />
[2]. J Control Release. 2015 Nov 10;217:337-44. doi: 10.1016/j.jconrel.2015.08.051. Epub 2015 Sep 3.<br />
N(1)-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice.<br />
Andries O(1), Mc Cafferty S(1), De Smedt SC(2), Weiss R(3), Sanders NN(4), Kitada T(5).<br />
Author information: (1)Laboratory of Gene Therapy, Department of Nutrition, Genetics and Ethology, Faculty of Veterinary Medicine, Ghent University, Heidestraat 19, B-9820 Merelbeke, Belgium. (2)Laboratory of General Biochemistry and Physical Pharmacy, Ghent Research Group on Nanomedicine, Faculty of Pharmaceutical Sciences, Ghent University, Harelbekestraat 72, B-9000 Ghent, Belgium. (3)Synthetic Biology Center, Department of Biological Engineering, Massachusetts Institute of Technology, 500 Technology Square, Cambridge, MA, USA. (4)Laboratory of Gene Therapy, Department of Nutrition, Genetics and Ethology, Faculty of Veterinary Medicine, Ghent University, Heidestraat 19, B-9820 Merelbeke, Belgium. Electronic address: [email protected]. (5)Synthetic Biology Center, Department of Biological Engineering, Massachusetts Institute of Technology, 500 Technology Square, Cambridge, MA, USA. Electronic address: [email protected].<br />
Messenger RNA as a therapeutic modality is becoming increasingly popular in the field of gene therapy. The realization that nucleobase modifications can greatly enhance the properties of mRNA by reducing the immunogenicity and increasing the stability of the RNA molecule (the Kariko paradigm) has been pivotal for this revolution. Here we find that mRNAs containing the N(1)-methylpseudouridine (m1Ψ) modification alone and/or in combination with 5-methylcytidine (m5C) outperformed the current state-of-the-art pseudouridine (Ψ) and/or m5C/Ψ-modified mRNA platform by providing up to ~44-fold (when comparing double modified mRNAs) or ~13-fold (when comparing single modified mRNAs) higher reporter gene expression upon transfection into cell lines or mice, respectively. We show that (m5C/)m1Ψ-modified mRNA resulted in reduced intracellular innate immunogenicity and improved cellular viability compared to (m5C/)Ψ-modified mRNA upon in vitro transfection. The enhanced capability of (m5C/)m1Ψ-modified mRNA to express proteins may at least partially be due to the increased ability of the mRNA to evade activation of endosomal Toll-like receptor 3 (TLR3) and downstream innate immune signaling. We believe that the (m5C/)m1Ψ-mRNA platform presented here may serve as a new standard in the field of modified mRNA-based therapeutics.<br />
DOI: 10.1016/j.jconrel.2015.08.051 PMID: 26342664 [Indexed for MEDLINE]<br />
<br />
[3]. Science. 2021 Jan 8;371(6525):145-153. doi: 10.1126/science.aay3638.<br />
A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis.<br />
Krienke C(1)(2), Kolb L(#)(1), Diken E(#)(1), Streuber M(1), Kirchhoff S(1), Bukur T(1), Akilli-Öztürk Ö(1), Kranz LM(3), Berger H(3), Petschenka J(1)(4), Diken M(1)(3), Kreiter S(1)(3), Yogev N(5)(6), Waisman A(2)(5), Karikó K(3), Türeci Ö(3)(7), Sahin U(8)(2)(3).<br />
Author information: (1)TRON – Translational Oncology at the University Medical Center of the Johannes Gutenberg University gGmbH, Freiligrathstr. 12, Mainz 55131, Germany. (2)Research Center for Immunotherapy (FZI), University Medical Center at the Johannes Gutenberg University, Langenbeckstr. 1, Mainz 55131, Germany. (3)Biopharmaceutical New Technologies (BioNTech) Corporation, An der Goldgrube 12, Mainz 55131, Germany. (4)Cancer Immunology and Immune Modulation, Boehringer Ingelheim Pharma GmbH & Co. KG, Birkendorfer Str. 65, 88397 Biberach an der Riss, Germany. (5)Institute for Molecular Medicine, University Medical Center of the Johannes Gutenberg University, Mainz 55131, Germany. (6)Clinic and Polyclinic for Dermatology and Venereology, University Hospital Cologne, Kerpenerstr. 62, Cologne 50937, Germany. (7)CI3 – Cluster for Individualized Immunointervention e.V., Hölderlinstraße 8, 55131 Mainz, Germany. (8)TRON – Translational Oncology at the University Medical Center of the Johannes Gutenberg University gGmbH, Freiligrathstr. 12, Mainz 55131, Germany. [email protected]. (#)Contributed equally<br />
Comment in Nat Rev Immunol. 2021 Feb;21(2):72. Nat Rev Drug Discov. 2021 Feb;20(2):99. Mol Ther. 2021 Mar 3;29(3):896-897. Nat Biotechnol. 2021 Apr;39(4):419-421.<br />
The ability to control autoreactive T cells without inducing systemic immune suppression is the major goal for treatment of autoimmune diseases. The key challenge is the safe and efficient delivery of pharmaceutically well-defined antigens in a noninflammatory context. Here, we show that systemic delivery of nanoparticle-formulated 1 methylpseudouridine-modified messenger RNA (m1Ψ mRNA) coding for disease-related autoantigens results in antigen presentation on splenic CD11c+ antigen-presenting cells in the absence of costimulatory signals. In several mouse models of multiple sclerosis, the disease is suppressed by treatment with such m1Ψ mRNA. The treatment effect is associated with a reduction of effector T cells and the development of regulatory T cell (Treg cell) populations. Notably, these Treg cells execute strong bystander immunosuppression and thus improve disease induced by cognate and noncognate autoantigens.<br />
DOI: 10.1126/science.aay3638 PMID: 33414215 [Indexed for MEDLINE]<br />
<br />
[4]. J Control Release. 2015 Nov 10;217:345-51. doi: 10.1016/j.jconrel.2015.08.007. Epub 2015 Aug 8.<br />
Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes.<br />
Pardi N(1), Tuyishime S(1), Muramatsu H(1), Kariko K(1), Mui BL(2), Tam YK(2), Madden TD(2), Hope MJ(2), Weissman D(3).<br />
Author information: (1)Department of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA. (2)Acuitas Therapeutics, Vancouver, V6T 1Z3 BC, Canada. (3)Department of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA. Electronic address: [email protected].<br />
In recent years, in vitro transcribed messenger RNA (mRNA) has emerged as a potential therapeutic platform. To fulfill its promise, effective delivery of mRNA to specific cell types and tissues needs to be achieved. Lipid nanoparticles (LNPs) are efficient carriers for short-interfering RNAs and have entered clinical trials. However, little is known about the potential of LNPs to deliver mRNA. Here, we generated mRNA-LNPs by incorporating HPLC purified, 1-methylpseudouridine-containing mRNA comprising codon-optimized firefly luciferase into stable LNPs. Mice were injected with 0.005-0.250mg/kg doses of mRNA-LNPs by 6 different routes and high levels of protein translation could be measured using in vivo imaging. Subcutaneous, intramuscular and intradermal injection of the LNP-encapsulated mRNA translated locally at the site of injection for up to 10days. For several days, high levels of protein production could be achieved in the lung from the intratracheal administration of mRNA. Intravenous and intraperitoneal and to a lesser extent intramuscular and intratracheal deliveries led to trafficking of mRNA-LNPs systemically resulting in active translation of the mRNA in the liver for 1-4 days. Our results demonstrate that LNPs are appropriate carriers for mRNA in vivo and have the potential to become valuable tools for delivering mRNA encoding therapeutic proteins.<br />
DOI: 10.1016/j.jconrel.2015.08.007 PMCID: PMC4624045 PMID: 26264835 [Indexed for MEDLINE]<br />
<br />
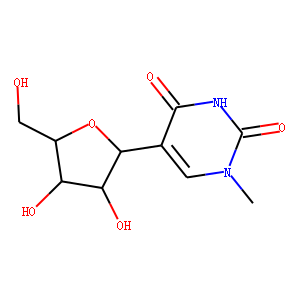
[5]. J Antibiot (Tokyo). 1976 Aug;29(8):818-23. doi: 10.7164/antibiotics.29.818.<br />
1-methylpseudouridine, a metabolite of Streptomyces platensis.<br />
Argoudelis AD, Mizsak SA.<br />
1-Methylpseudouridine is a new metabolite isolated from culture filtrates of Streptomyces platensis. The structure of this compound was determined from its physical and spectral properties.<br />
DOI: 10.7164/antibiotics.29.818 PMID: 993120 [Indexed for MEDLINE]
|