What is the Principle behind Microwave-accelerated rDA Reactions?
Abstract
The differences between microwave heating and conventional heating methods in organic chemical synthesis had been discussed in this paper. By studying the factors influencing reaction rates under microwave and conventional heating, such as reactant concentration, solvent viscosity, and reactant polarity, the existence of selective microwave heating has been demonstrated, providing theoretical guidance for designing microwave radiation-assisted reactions. The study focuses on the retro-Diels-Alder reaction and compares the conversion rates of different substrates under microwave and conventional heating conditions. These findings emphasize the importance of further research to understand the complex factors affecting microwave heating reactions.
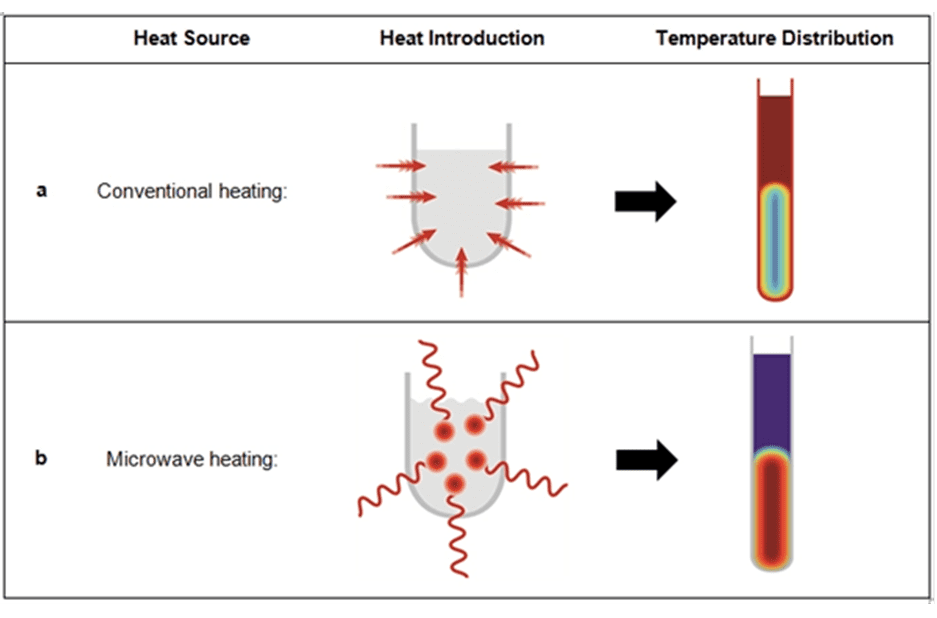
When it comes to microwaves, the first thing that most people think of is a microwave oven, which is very convenient for heating up food or even cooking noodles (Safety tip: Do not microwave eggs. Don’t ask me how I know~). Microwaves, which are electromagnetic waves with short wavelengths and high frequencies, do not generate heat themselves. Instead, they heat up substances (such as food) by interacting with polar molecules in the medium. Under the influence of microwaves, polar molecules polarize and continuously change their orientation with the polarity of the electromagnetic field. This generates high-frequency oscillations and friction among the molecules, converting electromagnetic energy into heat energy.
In recent years, many chemists have applied this effect to organic chemical synthesis. This approach not only avoids heat conduction between the heat source and the reaction vessel in conventional heating processes (CH), but also enables rapid and uniform heating. However, the mechanism of microwave-assisted accelerated reactions is still not fully understood and requires further research for clarification.
In 2014, Professor Gregory B. Dudley and his research team at West Virginia University in the United States proposed that microwave radiation can induce selective heating of specific reaction components, leading to accelerated processes that cannot be achieved with conventional heating methods (J. Org. Chem., 2014, 79, 7425-7436; J. Org. Chem, 2014, 79, 7437-7450). This is because under microwave radiation, molecules with higher polarity are more easily heated, resulting in different heating efficiencies. Recently, they investigated the retro-Diels-Alder (rDA) reaction between anthracene and fumaric acid derivatives at high temperatures by designing reactants with different polarities. They used this study to explore the relevant mechanisms of microwave-assisted accelerated reactions.
Compared to conventional heating methods, microwave heating significantly increases the rate constant of the reaction, especially for reactants with larger molecular polarities. Additionally, changing the concentration of reactant molecules and the viscosity of the reaction solvent can also affect the rate constant under microwave heating conditions, whereas it has no impact on traditional heating methods. These results provide ample evidence for the selective heating of microwaves in organic reactions. The related work was published in the journal Chemical Communications.
The retro-Diels-Alder reaction involved in this study is illustrated in the figure below. The reason for choosing this reaction as a model is that the reactant 1 is theoretically more polar than the products 2 and 3, which facilitates the selective microwave heating of reactant 1. Additionally, fumaric acid derivatives are easy to synthesize and modify, which is advantageous for adjusting their solubility in non-polar solvents. Furthermore, the authors used a weakly polar tridecane as the reaction solvent. This not only promotes the aggregation of reactants in solution but also facilitates the rapid heating of these small regions of reactants by microwaves.
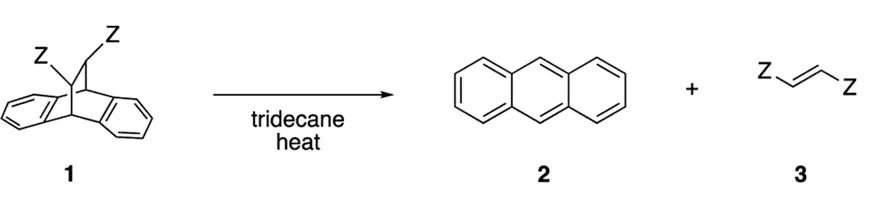
Fig 1. The retro-Diels-Alder reaction (Z represents carboxylic acid, amide, ester, etc.)
At a reaction temperature of 200°C, reactant 1 decomposes into non-polar anthracene 2 and alkene product 3. Compared to conventional heating methods, the reaction rate is significantly increased when using microwave radiation for heating. Particularly, when the substrate is 4, the first-order rate constant increases by nearly 32% (0.94×10-2 vs. 0.71×10-2 min-1, as shown in the figure below). As shown in Figure 3, at the same temperature, the two heating methods exhibit significantly different first-order rate constants. This could be attributed to the selective heating of certain polar molecules under microwave heating conditions, where the temperature detected macroscopically does not necessarily reflect the actual reaction temperature. By back-calculating the reaction temperature based on the reaction rate constant (0.94×10-2 min-1), the authors found that the actual reaction temperature under microwave heating conditions should be 204°C.
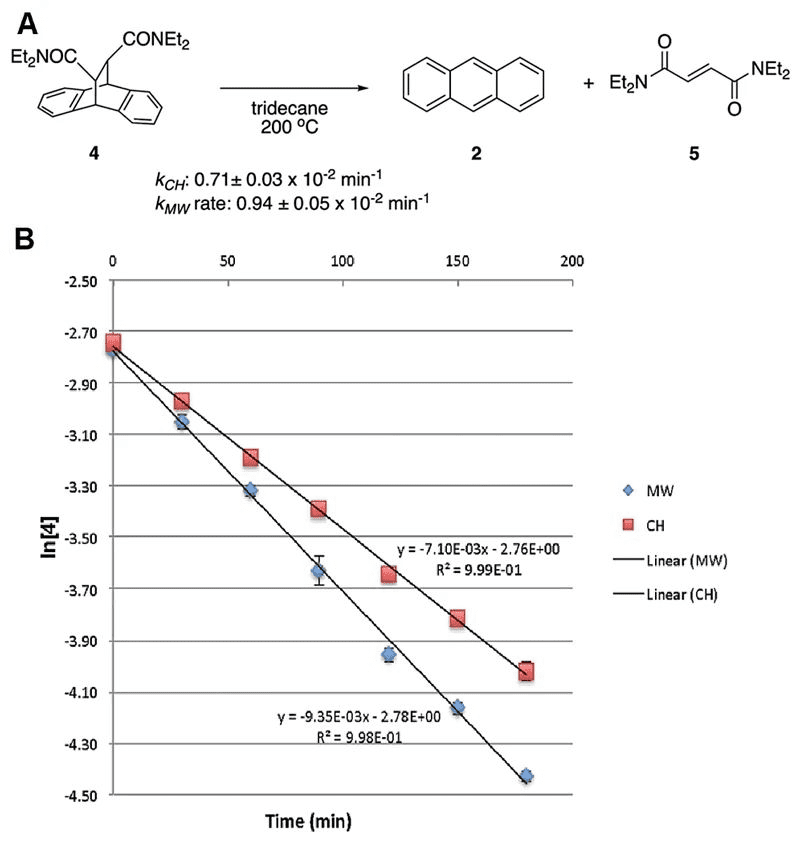
Fig 2. The mechanism of the retro-Diels-Alder reaction of substrate 4 and its first-order rate constant.
Subsequently, the authors investigated the effects of surfactants, reactant concentration, and solvent viscosity on the microwave heating process. As shown in Fig 3, when Span-60 was added to the reaction, there was a slight change in the reaction rate during the microwave heating process (1.07×10-2 min-1 vs. 0.94×10-2 min-1), while there was no change observed in the conventional heating process. When the reactant concentration was decreased (0.065 M to 0.020 M), the reaction rate in the microwave heating process increased (0.94×10-2 min-1 to 1.0×10-2 min-1), whereas the reaction rate in the conventional heating process remained unchanged (0.72×10-2 min-1). When a solvent with higher viscosity (heptadecane) was used for the reaction, the reaction rate significantly increased during the microwave heating process (0.74 to 1.21×10-2 min-1, a 66% increase), while the reaction rate in the conventional heating process remained almost unchanged. These results all indicate the existence of selective heating in the microwave heating process.
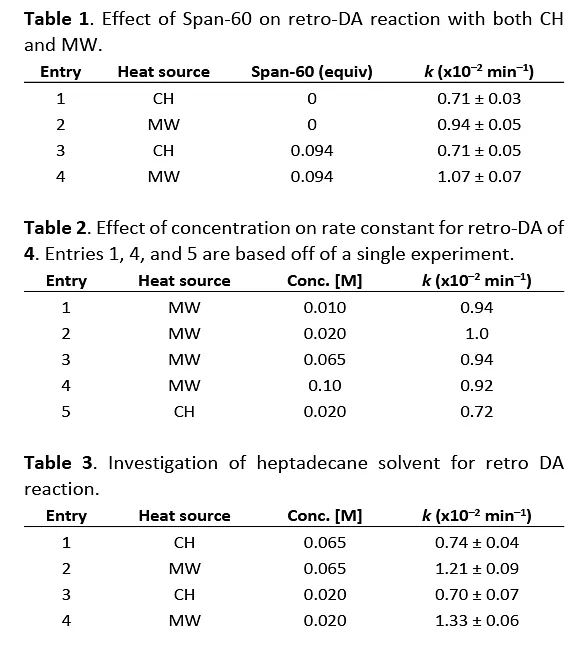
Fig 3. The effects of surfactants, reactant concentration, and solvent viscosity on both microwave and conventional heating processes.
Finally, the authors investigated the conversion rates of different retro-Diels-Alder reaction substrates under microwave and conventional heating conditions. After a 3-hour reaction under standard conditions, the increase in conversion rate for dimethyl ester 6 was the same for both microwave and conventional heating (60% vs. 51%), as was observed for the diimide 4 (81% vs. 72%). However, the increase in conversion rate for the dibenzyl ester 7 was larger (48% vs. 31%).
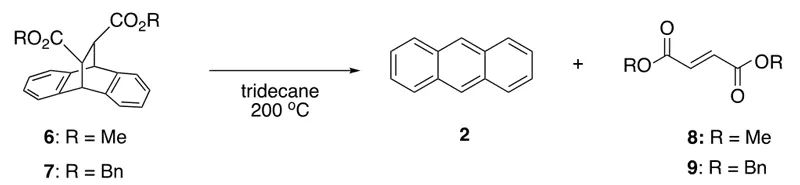

Fig 4. The conversion rates of different retro-Diels-Alder reaction substrates.
Conclusion
The difference between microwave heating and conventional heating methods lies in the fact that the former primarily interacts with polar molecules to generate heat. Due to the different polarities of molecules, their heating capabilities vary, thereby providing conditions for selective microwave heating. Through comparative analysis of factors affecting the reaction rates of conventional and microwave heating (such as reactant concentration, solvent viscosity, and reactant polarity), the authors have demonstrated the existence of selective microwave heating. This provides theoretical guidance for the design of new microwave radiation-assisted reactions and reaction devices. It is evident that there are many factors influencing the microwave heating reaction process, indicating the need for further detailed research.
Reference
Frasso, M. A., Stiegman, A. E., & Dudley, G. B. (2020). Microwave-specific acceleration of a retro-Diels–Alder reaction. Chemical Communications, 56(76), 11247-11250.