Ursodeoxycholic Acid- A New Covid Treatment
Abstract
Ursodeoxycholic acid(UDCA), chemical name 3α,7β-dihydroxy-5 β-cholestane-24-acid, is an odorless, organic compound(Scheme 1). In medicine, it is used to increase the secretion of bile acid, change the composition of bile, reduce cholesterol and cholesterol lipid in bile, and facilitate the gradual dissolution of cholesterol in gallstones. Clinically, it is used for the treatment of gallstones, cholestatic liver disease, fatty liver, various types of hepatitis, toxic liver disorders, cholecystitis, cholangitis and bile dyspepsia, bile reflux gastritis, eye diseases, etc.
SARS-CoV-2 infects the host by binding to ACE2
Coronaviruses are a large family of enveloped plus-strand RNA viruses, which generally infect humans and other mammals as alpha or beta coronaviruses. Bats are the most important animal reservoirs for coronavirus. Cross-species transmission of coronaviruses has led to three major outbreaks in the past 20 years, caused by SARS-CoV, MERS-CoV, and, most recently SARS-CoV-2. The specific recognition of host receptors by coronaviruses is usually mediated by spike proteins on their surfaces. Among the suppressed human coronavirus receptors, there are three transmembrane enzymes, namely ACE2, DPP4, and APN.
Early studies observed that ACE2 was localized mainly in the heart, kidney, and testis, and expressed at low levels in a variety of other tissues, especially the colon and lungs. Later studies also showed that ACE2 played an important role in other organs, such as the liver and intestine. ACE2 is usually localized in the lumen surface of epithelial cells, as opposed to ACE, which appears to be evenly distributed between the apical and basolateral membranes of polarized cells. However, when SARS Cov infects ACE2-expressing cell cavities, its infective efficacy increases by 10 times. Both ACE and ACE2 belong to the M2 family of metalloproteinases, whose active site domains are exposed to the extracellular surface and promote the metabolism of circulating peptides. Both ACE and ACE2 catalyze the reaction by using zinc, which coordinates with the conserved histidine in the active site to promote the nucleophilic attack of water molecules on the carbonyl bond of the substrate and form non-covalently bound intermediates.
UDCA affects viral infection by regulating ACE2 through FXR
Ursodeoxycholic acid(UDCA), chemical name 3α,7β-dihydroxy-5 β-cholestane-24-acid, is an odorless, organic compound(Scheme 1). In medicine, it is used to increase the secretion of bile acid, change the composition of bile, reduce cholesterol and cholesterol lipid in bile, and facilitate the gradual dissolution of cholesterol in gallstones. Clinically, it is used for the treatment of gallstones, cholestatic liver disease, fatty liver, various types of hepatitis, toxic liver disorders, cholecystitis, cholangitis and bile dyspepsia, bile reflux gastritis, eye diseases, etc.
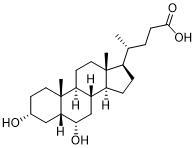
Scheme 1 The structure of UDCA
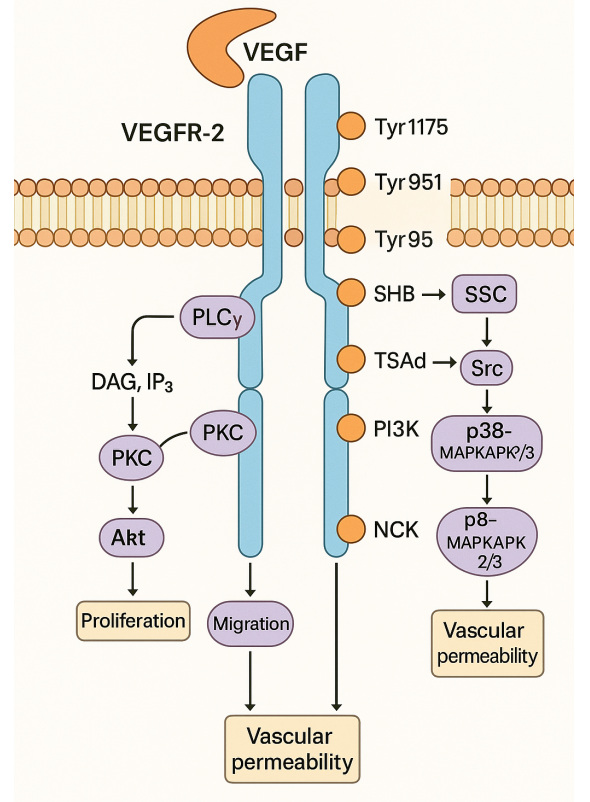
Recent experiments have shown that FXR is activated in CDCA-treated cells, and downstream signal ACE2 is increased. FXR5 signaling was affected in UDCA-treated cells, and the level of ACE2 in the upper respiratory tract and intestine was significantly reduced (Figure 1). These experimental data indicate that FXR can regulate the expression of ACE2 in the respiratory tract and intestine, and UDCA can effectively reduce ACE2 level through FXR.
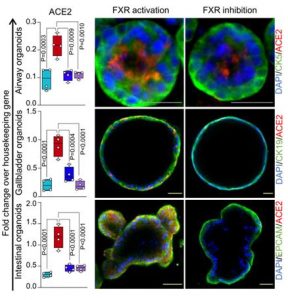
Figure 1 FXR modulates ACE2 expression
The team then ran a hamster simulation with nine animals treated with UDCA for seven days and six as a control group (both groups were not infected with the virus). Subsequently, each infected animal was co-housed with a group of n=3 randomly selected sentinel (uninfected) hamsters from the UDCA or control group for a period of 4 days to judge SARS-CoV-2 transmission. Viral infection in sentinel animals was assessed with plaque assays from the animal lungs harvested at the end of the experiment and confirmed with daily swabs and viral QPCR in tissue harvested from the lungs and nasal turbinates of the animals at the end of the experiment. Our data demonstrate that UDCA treatment prevented transmission of SARS-CoV-2 in n=6 out of 9 sentinel animals (33% infected vs. 67% uninfected); while SARS-CoV-2 was transmitted in n=6 out of 6 (100%) sentinel hamsters receiving vehicle (P=0.027; Fisher’s exact test) for the duration of the experiment (Figure 2). The experimental results showed that the hamsters’ ability to be infected with UDCA was significantly weakened, and UDCA had a certain preventive effect on SARS-CoV-2.
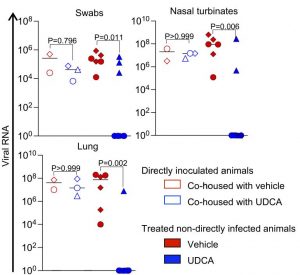
Figure 2 FXR inhibition reduces ACE2 and SARS-CoV-2 infection in vivo
Together, these results validate UDCA as a new platform for disease modeling and drug testing against SARS-CoV-2 infection; FXR has been identified as a new therapeutic target for the management of COVID-19 and the prevention of SARS-CoV-2 infection through FXR regulation of ACE2 as well as infection by other viruses that infect the host via ACE2.